Ca2+ ions dosage with a selective electrode (potentiometry)
Electrode constitution
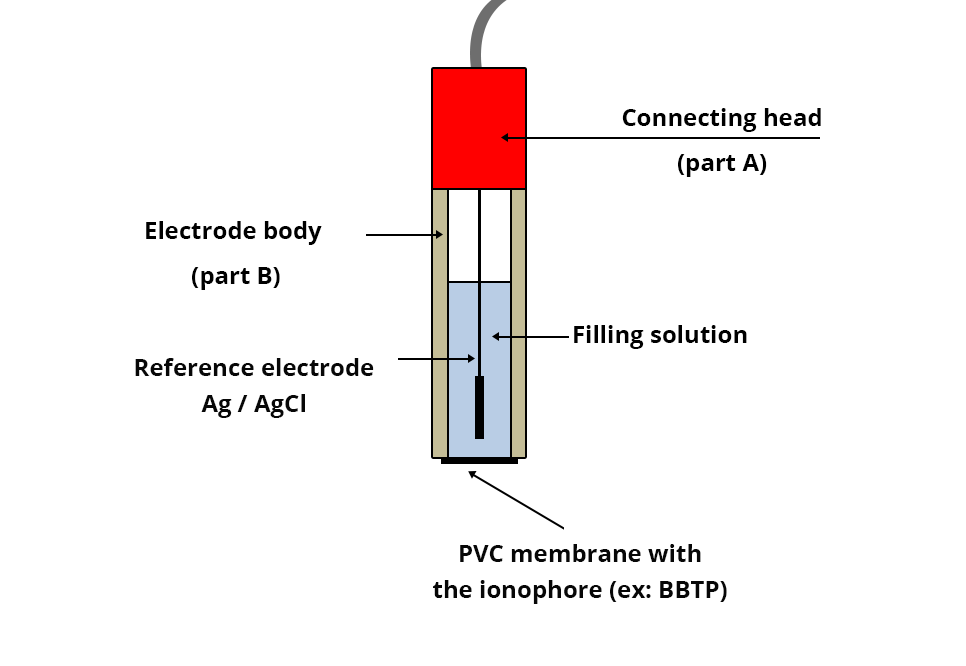
This electrode consists of:
- An Ag/AgCl (chlorinated silver wire) reference electrode wired through a connecting head (part A) to an electric wire;
- A plastic body with a PVC (polyvinyl chloride) membrane at its end containing an ionophore capable of almost selectively complexing Ca2+ ions (active part of the electrode);
- An internal KNO3 filling solution supplied by the manufacturer.