Determination of calcium and magnesium ions by colorimetric titration:
Dosage principle
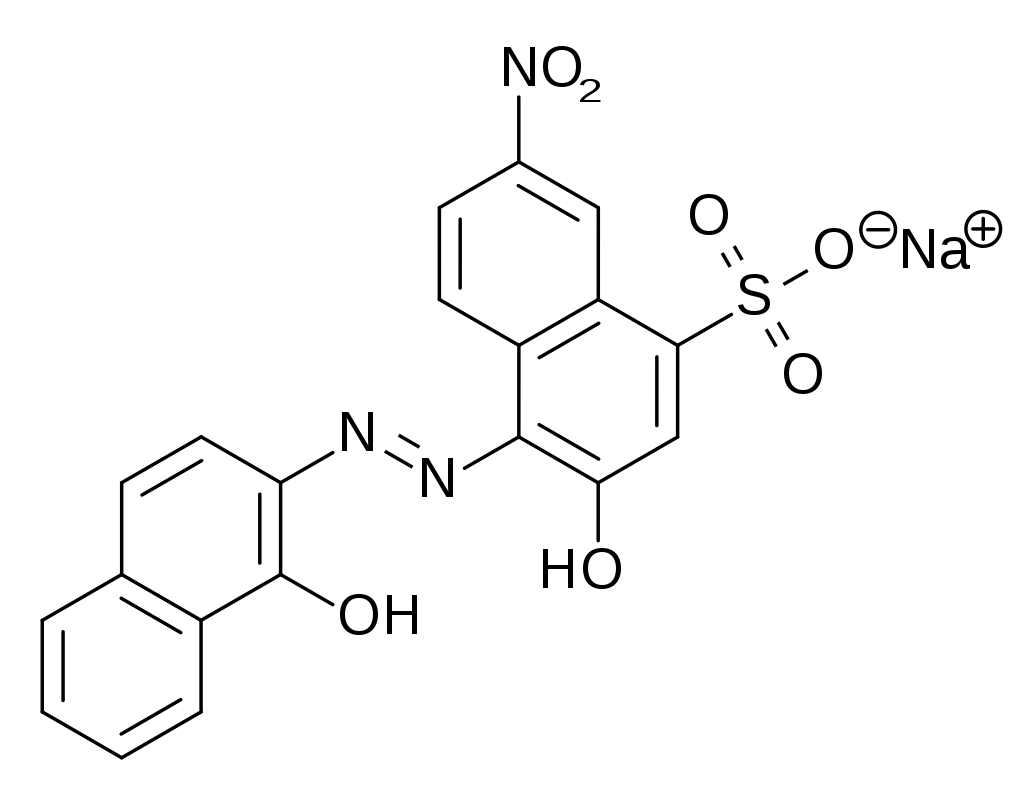
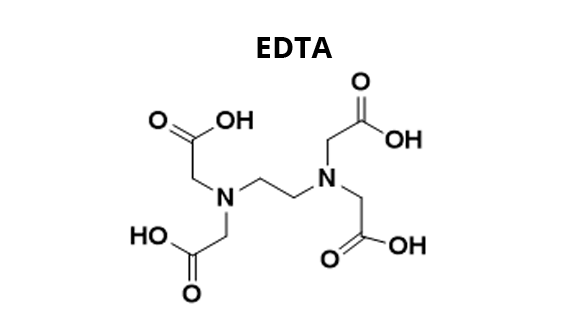
The determination of water hardness is based on the addition of eriochrome black T (EBT) to the water to be analyzed to form Mg-EBT and Ca-EBT complexes (1). The solution takes on a red-purple color characteristic of these complexes. We add a buffer solution to work at pH 10. The solution obtained is then titrated with ethylene diamine tetraacetic acid (EDTA). First, Mg-EDTA and Ca-EDTA complexes are formed with free Mg2+ and Ca2+ ions (i.e. not complexed with EBT) (2) and then the ions already complexed with EBT interact with EDTA (3). This releases EBT in the solution and gives it a blue color, characteristic of this molecule at pH 10.
(1) Mg2+(aq) + EBT(aq) = Mg-EBT(aq) and Ca2+(aq) + EBT(aq) = Ca-EBT(aq)
(2) Mg2+(aq) + EDTA(aq) = Mg-EDTA(aq) and Ca2+(aq) + EDTA(aq) = Ca-EDTA(aq)
(3) Mg-EBT(aq) + EDTA(aq) = Mg-EDTA(aq) + EBT(aq) and Ca-EBT(aq) + EDTA(aq) = Ca-EDTA(aq) + EBT(aq)
(2) Mg2+(aq) + EDTA(aq) = Mg-EDTA(aq) and Ca2+(aq) + EDTA(aq) = Ca-EDTA(aq)
(3) Mg-EBT(aq) + EDTA(aq) = Mg-EDTA(aq) + EBT(aq) and Ca-EBT(aq) + EDTA(aq) = Ca-EDTA(aq) + EBT(aq)
Data:
pKS (Ca(OH)2) = 5.26; pKS (Mg(OH)2) = 9.2; logβ (Ca-EBT)= 5.4; logβ (Mg-EBT)= 7.0; logβ (Ca-EDTA)= 10.7; logβ (Mg-EDTA)= 8.6.