How to perform a TLC?
Experiment B: influence of the presence of absorbent group under UV-visible light
In different vials insert some of the following solutions:
6- Ferrocene (in CH2Cl2)
7- PhCHO (in CH2Cl2)
8- Anthracene (in CH2Cl2)
9- Linalool (in CH2Cl2)
Using capillaries, apply each solution to the same TLC plate.
Observe using the two wavelengths of the lamp.
Place this CCM plate in the iodine chamber or in the KMnO4 chamber (then heat the plate with a hair dryer in the case of KMnO4).
Leave for few minutes.
Observe.

a) observed under UV
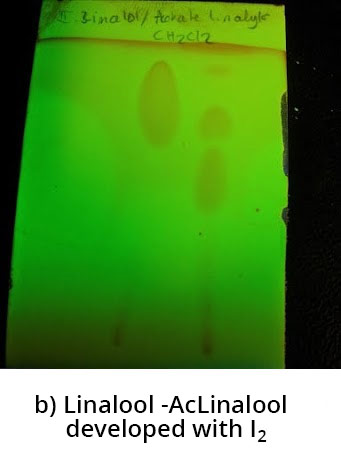
b) then after iodine development


Under UV: no spot is observed for linalool which does not have any absorbent sub-structure under UV; A spot of Rf close to 1 appears for linalool acetate.
After revelation with iodine a spot is observed for linalool - the iodine reveals the presence of this compound; other spots are also observed in linalool acetate.
The revelation by immersion in KMNO4 makes it possible to see the three compounds. The presence of impurities in linalool and linalool acetate is also observed.