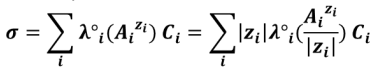Titrations
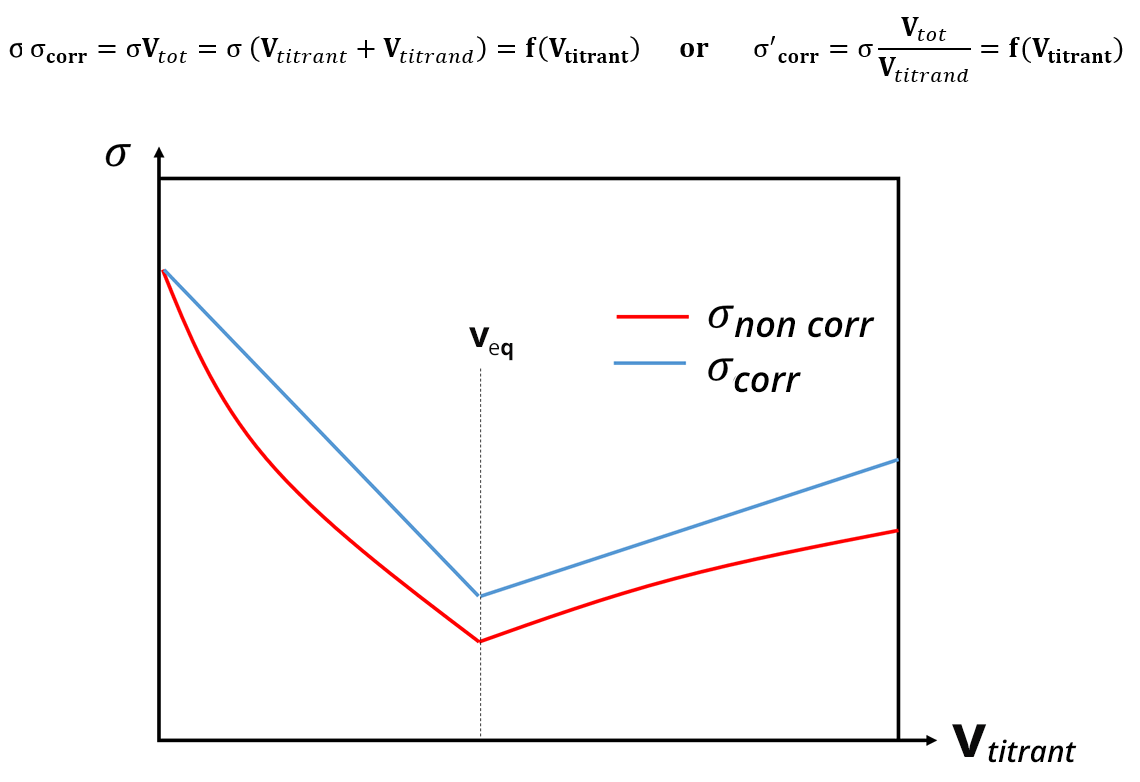
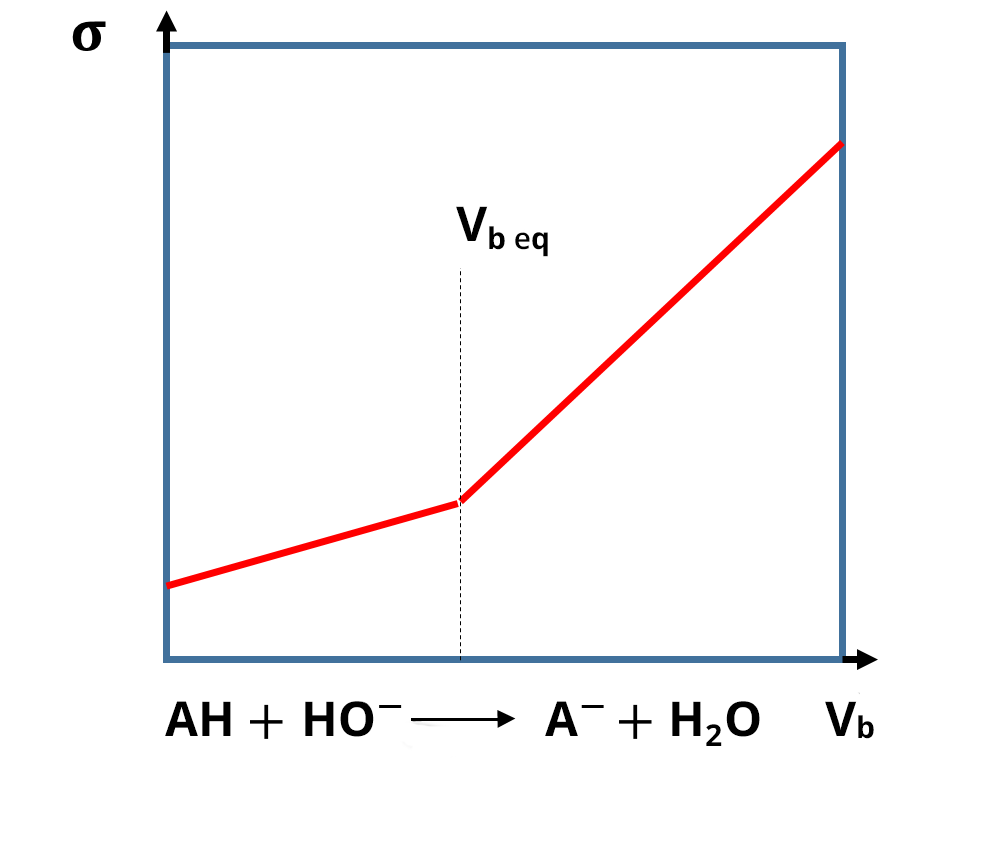
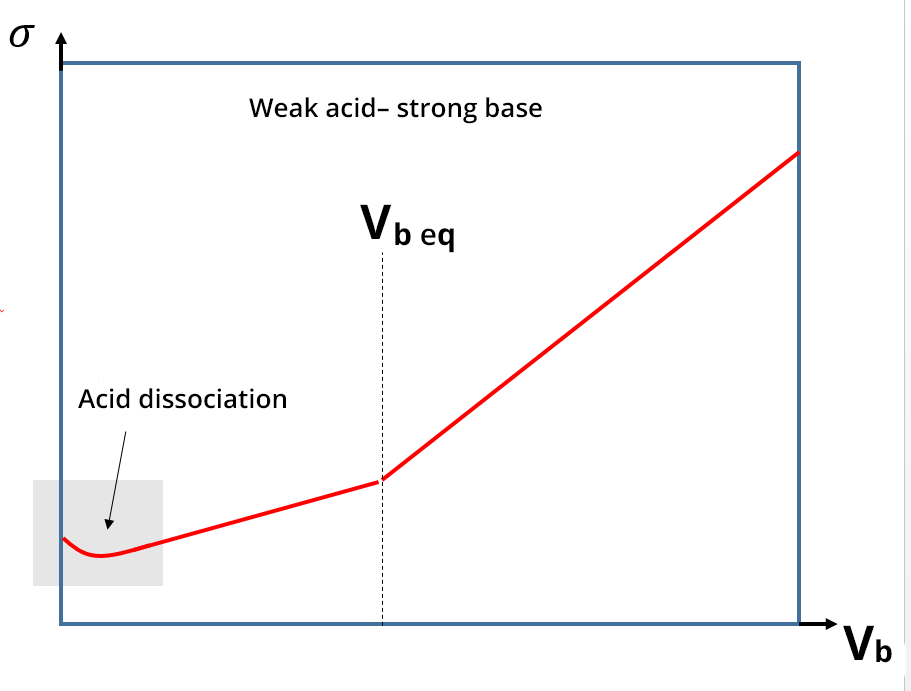
For conductimetric titrations, the volume V of the titrant is shown on the abissca (x-axis) and the conductance G (in S or mS) or the conductivity σ (in S. cm-1 or S.m-1) is shown on the ordinate (y-axis).
In the case of a titration, the calibration phase of the conductivity meter is not necessary, because the focus is on the development of the conductivity and not necessarily on its absolute value. The equivalence point is identified thanks to a break in the slope and the absence of calibration only shifts the y value: this does not change the value of the equivalent volume. In practice, the linear sections far from the equivalence point are the ones which are extended and used to identify the equivalence points.