What is conductivity?
Conductivity (noted σ) quantifies the ability of a solution to conduct electrical current (i.e. to allow electrical charges to move freely). This is directly related to the mobility (noted ui) of the ions present in the solution, which is the concentration-independent part of the conductivity.
For a sphere-shaped ion, mobility follows the subsequent relationship according to Stoke’s theory:
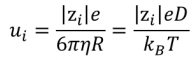
With zi the charge of the ion, e the elementary charge η the viscosity, R the radius of the solvated ion (hydrodynamic radius), kB the Boltzmann constant, D the diffusion coefficient and T the temperature. Mobility ui has for unit m2.s-1.V-1.
Thus:
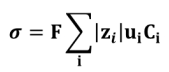
with F the Faraday constant in C.mol-1 (charge of one mole of elementary charges) and Ci the concentration in mol.m-3.
 Ci in mol.m-3 = 103Ci en mol.L-1
Ci in mol.m-3 = 103Ci en mol.L-1
For each ion, the ionic molar conductivity λi (AiZi) is defined by:
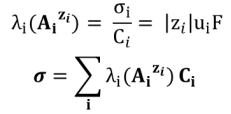
For polycharged ions, we most often use the ionic molar conductivity per mole of charge (also called equivalent or specific ionic conductivity)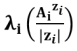 ) :
) :
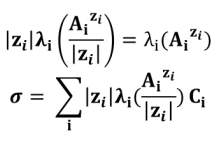
When C tends to 0 (infinite dilution), a new quantity is introduced: the ionic limiting molar conductivity (λ°) : λ°i = limCi → 0 λi. Typically in a diluted solution it is considered that :
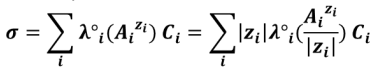
This is sometimes called Kohlrausch’s law.





