Values of molar ionic conductivity at 25°C
The more voluminous an ion is, the lower its mobility, and the lower its conductivity. It therefore follows that metallic cations usually have relatively low λ°i.
Be careful not to jump to conclusions because, in solution, ions are solvated (presence of a solvation shell around the ions): the smaller and the more charged the ion, the bigger the solvation shell. This explains, for example, the development of conductivity in the case of halide anions and alkali cations:
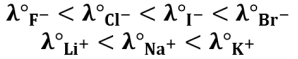
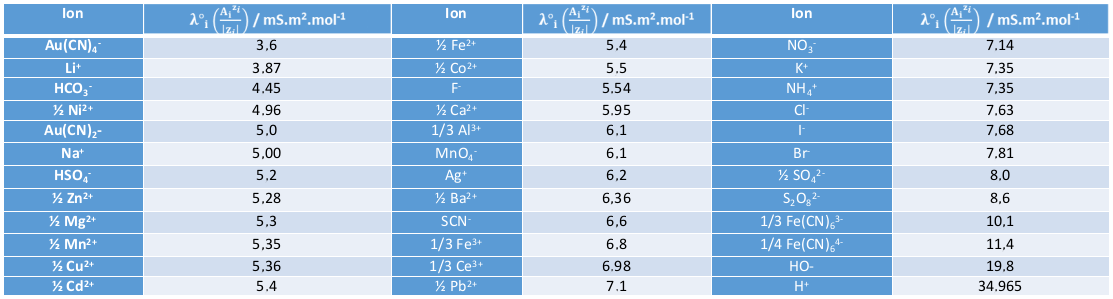
H+ and HO- ions have the highest conductivities because their mobility is very different from that of other ions. Indeed, unlike other ions, they do not move entirely in solution to carry their charge: they move from one place to another via a network of hydrogen bonds: this is called the Grotthuss mechanism.





