Basics of HPLC
Exercise 3: Analysis of nitrogen compounds
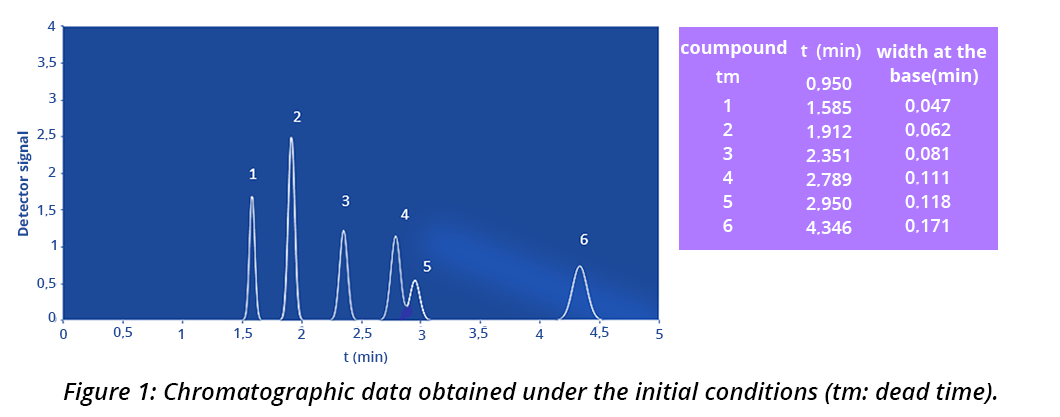
The separation of different nitrogen compounds is carried out on a stationary phase of C18-grafted silica (see Figure 1).
The dimensions of the column are as follows: L = 150 mm, i.d. = 4.6 mm, dp = 5 μm.
Initial conditions:
The mobile phase is a mixture of acetonitrile (ACN) and H2O in 75/25 proportions. The flow rate of the mobile phase is 1 mL/min. Detection is performed using a UV detector at a wavelength λ = 220 nm and the temperature of the column is kept constant at 30°C.
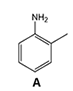
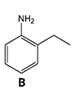
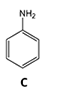
3)Chromatographic peaks 3, 4 and 6 are derived from aniline derivatives or aniline. Based on the structures of these molecules (Figure 2) and chromatographic conditions, associate (by dragging and dropping the molecules into the correct boxes) a compound with each of the chromatographic peaks 3, 4 and 6.
1)The chromatography used for this separation is:
reversed phase polarity partition chromatography See the “A little theory” tab of this sheet.
normal phase polarity partition chromatography
2)For this chromatography do the retention times depend on the temperature?
Yes See the “Illustrating theory” tab of this sheet.
No
Determination of some chromatographic parameters
1)For peak 5 of the chromatogram, calculate the retention factor (k) and the number of theoretical plates (N)
- Retention factor k(5):
k = 1.01
k = 2.11 See “To find out more”/“Chromatographic parameters” tabs of this sheet: tm = 0.95 min; tr(5) = 2.95 min, and width of the extrapolated base of the peak (I) = 0.118 min.
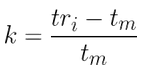 k= 2.11
k= 2.11k = 3.57
- Number of theoretical plates N(5):
N = 15,216
N = 8,887
N = 10,000
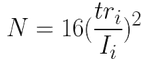 N = 10,000
N = 10,0002)After finding the least resolved pair of compounds on the chromatogram, calculate the resolution between the two peaks.
- The least resolved pair of compounds:
Pair of compounds 3,4
Pair of compounds 5,6
Pair of compounds 4,5
- Resolution between the compounds of the limiting pair:
R = 3.7
R = 1.50
R = 1.41
R = 1.00
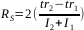
In order to optimize the chromatographic analysis, various parameters are modified one by one, all the other initial chromatographic conditions remaining unchanged.
7)From the parameters you have just determined, what is the best optimized chromatogram?
A
B
C Best resolution between peaks 4,5 and shortest analysis time.
8)Assign the previous chromatograms A, B and C to the changes in chromatographic conditions proposed below (all other chromatographic conditions remaining unchanged from initial conditions). It is assumed that the porosity of the column remains constant between the columns.
- The column temperature ature decreases to 25°C
A
B The chromatography involved is called partitioning. The retention times are dependent on the temperature. The drop in temperature leads to an increase in retention times. See the “Illustrating Theory” tab of this sheet.
C
- The particle size is reduced to 3 µm
A The number of theoretical plates increases, the efficiency is better. In addition, changing the particle size has no influence on the dead time if the porosity remains constant.
B
C
- column length and particle size have decreased
A
B
C In this case the tm and the tr are shorter (length of the column) and N is larger (better efficiency because the particles’ diameter is smaller).




