The pump: the beginning of the mobile phase
HPLC pumps play a major role. They allow the mobile phase to circulate throughout the entire system. They also control the composition of the mobile phase through several operating modes.
The mobile phase
In HPLC, the mobile phase composition is essential to encourage interactions with the column or, on the contrary, to favor the elution of compounds.
The majority of HPLC instruments can either maintain the mobile phase composition throughout the analysis, i.e. isocratic mode, or change the mobile phase composition during the analysis, i.e. gradient mode.
In reverse-phase partition chromatography (which is the most widely used), the solvents used are often an aqueous phase (called solvent A) and an organic phase (called solvent B).
Do you know why it is preferable that the bottle of the aqueous phase be amber? Discover the answer by clicking here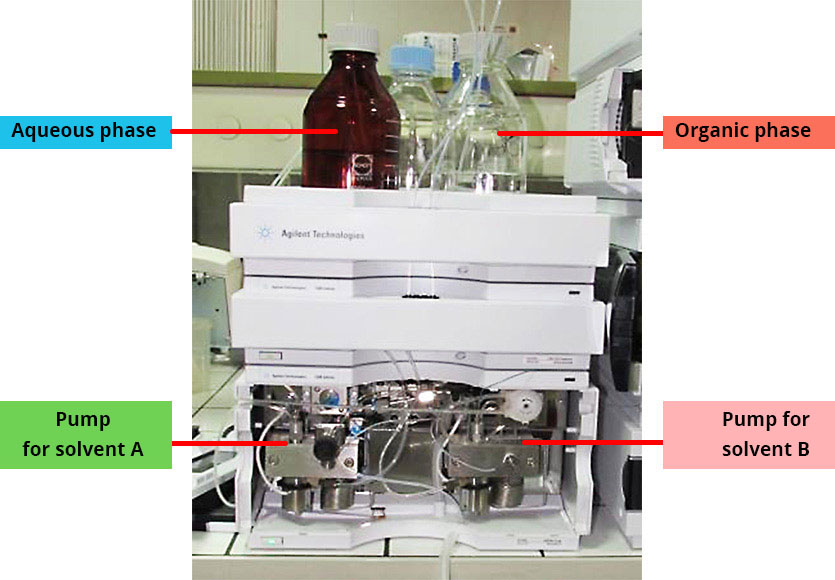
mobile phase composition
Unlike GC, in HPLC the mobile phase plays a major role in the migration of the compounds. So it is crucial to choose the correct mobile phase for the HPLC to succeed.
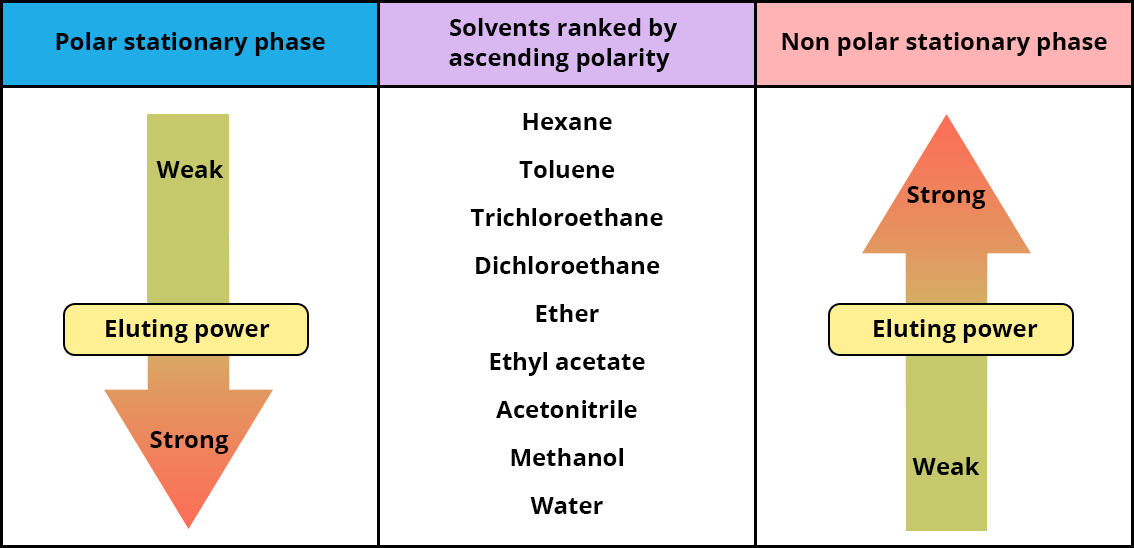
The eluting power of the solvent according to the type of liquid chromatography.
Since solvents must be used to assist the retention and/or elution of compounds in a chromatographic column to encourage the separation of compounds, it is essential to know the eluting power of the solvents most commonly used in HPLC.
The figure below classifies the different organic solvents according to their increasing polarity and indicates their eluting power for the use of a normal phase separation or reversed phase separation.
The higher the eluting power, the more effective the solvent will be in eluting the compounds in the column
In the case of ion-exchange chromatography, the solvent used depends on the type of retention expected
- Anion retention: mixture of sodium hydrogen carbonate (NaHCO3) and sodium carbonate (Na2CO3) -> easily eliminated with an anionic suppressor (Na+ ion exchange against H+ and HCO3- + H+ = H2O + CO2)
- Cation retention: hydrochloric acid (HCl) -> easily eliminated with a cationic suppressor (Cl- replaced by OH- and H+ + OH- -> H2O)
Thus, thanks to these two mobile phases and the use of an ion suppressor, the ion analysis generally done with a conductometer is not disturbed by the presence of the ions of the mobile phase, thereby increasing the sensitivity of the analysis.
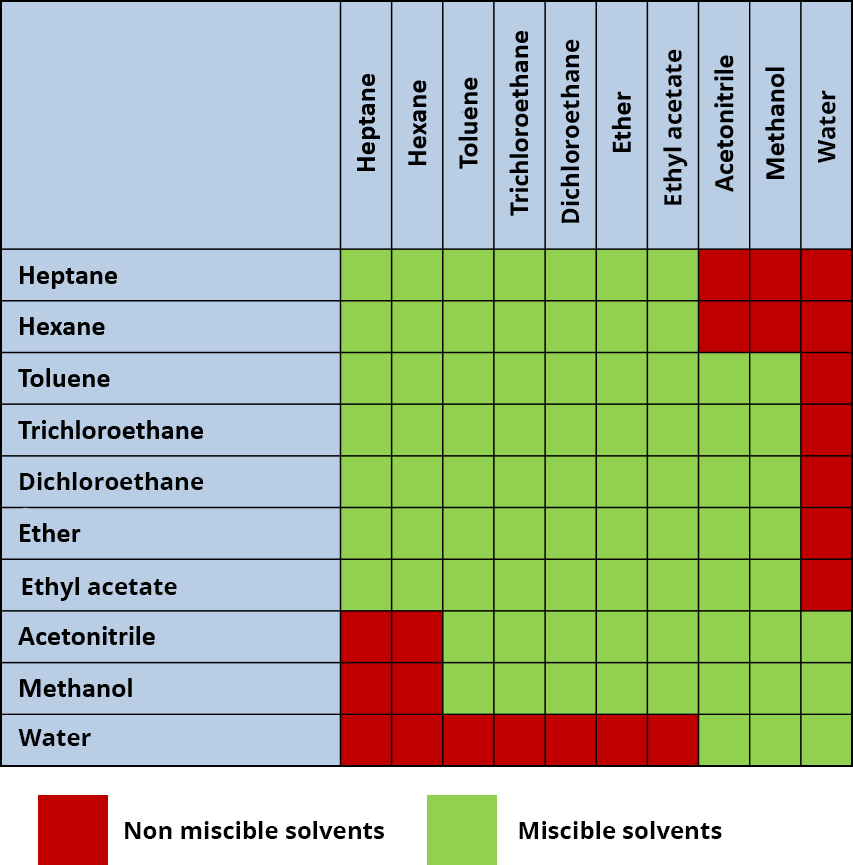
Go to the sheet "Chemicals"Solvent mixtures
In HPLC, it is possible to use only one solvent, but the majority of instruments use a mixture of solvents (either prepared beforehand, or automatically mixed by the pumps). Some instruments make mixtures with up to 4 solvents simultaneously.
Nevertheless, the use of several solvents requires ensuring their miscibility. Using two non-miscible solvents would indeed make the solvent mix inoperable and would highly disturb the retention of compounds in the column and the repeatability of the analyses.
The use of mobile phase additives
In some cases, solvents are not sufficient to ensure an acceptable chromatographic separation or correct analysis. It is therefore necessary to use additives that will modify the characteristics of the mobile phase and change the retention or analysis conditions.
Here are some cases of additive use in mobile phases:- pH-stabilization for the separation of acidic compounds: use of 0.1% formic acid. In this case, pH-stabilization for the separation of acidic compounds. As a result, like the pH In general the shape of the chromatographic peaks is greatly improved.
- Improvement of the analysis by mass spectrometry: use of 0.1% formic acid or 10 mM ammonium hydroxide (NH4OH) or ammonium formate (COONH4). These additives will improve the analysis of compounds by mass spectrometry by encouraging their ionization (cf, mass spectrometry).
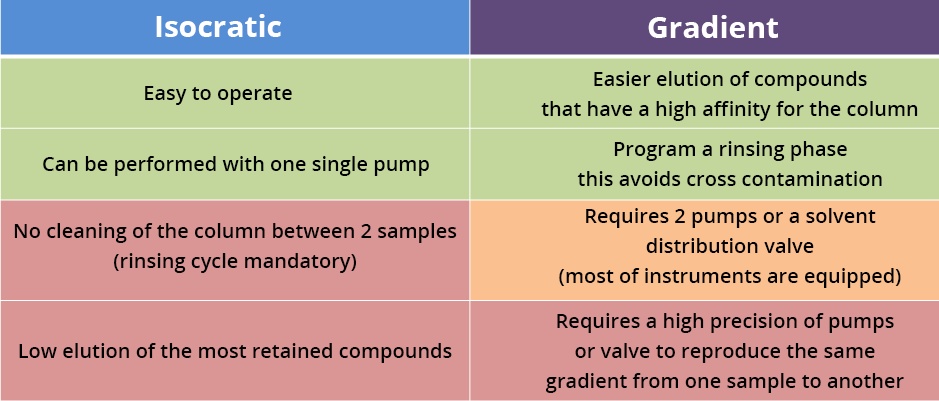
Isocratic or gradient elution
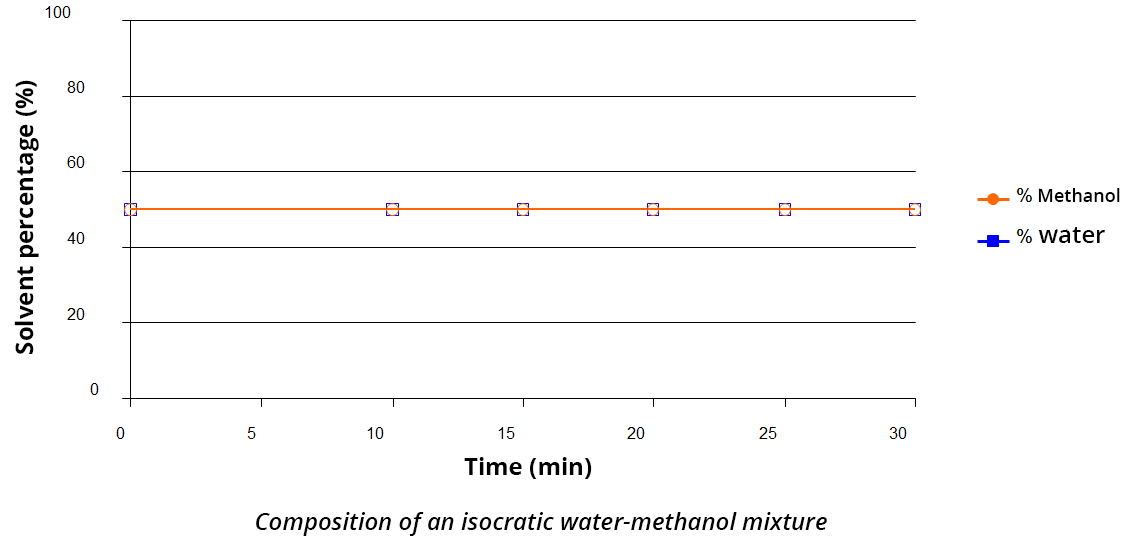
Isocratic elution
The mobile phase’s composition does not change during the analysis. If the mobile phase is a mixture, the percentage of each solvent in this mixture remains constant throughout the elution (see the mobile phase example with water/methanol 50/50 v/v).
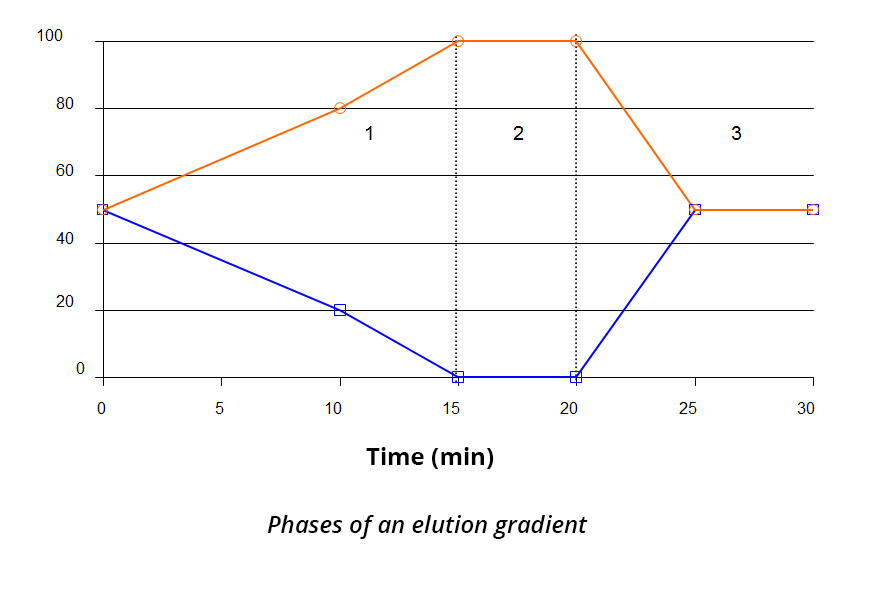
Elution gradient:
The mobile phase composition changes during the elution, allowing the separation of compounds that are hard to separate in isocratic mode.
This is generally carried out in 3 phases:
- Elution of the compounds
- Column rinsing
- Reconditioning
The elution gradient is particularly used for the analysis of complex samples and/or multi-compound analyses. The gradient not only makes it possible to neatly separate the different compounds to be eluted from the column, but also to thoroughly rinse the column between two injections to prevent the clogging of the stationary phase after injection.
To degas or not to degas?
Any solvent in contact with the atmosphere will dissolve gases (including oxygen) until the equilibrium point between the two phases is reached. Thus dissolved, the gases are likely to disturb the mixture of two solvents by generating microbubbles at high pressure that can disturb the analysis (retention and/or detection). Moreover, the presence of oxygen can lead to the oxidation of the metallic capillaries and the stationary phase of the column.
It is therefore important in chromatography to make sure that solvents contain no (or very little) dissolved gas when they are mixed in order to prevent the formation of microbubbles. To this end, two solutions are available:
- The instrument is equipped with an in-line vacuum degasser. The degasser is generally placed between the bottles of solvents and the pumps.
- By sparging or ultrasonic bath. Sparging is a process in which dissolved gases are replaced with an inert gas to prevent oxidation, whereas the ultrasonic bath allows for the volatization of dissolved gases. Ideally, after this type of degassing, the bottles must be kept under vacuum or in a controlled atmosphere (otherwise the gases in the atmosphere will dissolve again). Caution: the ultrasonic bath heats the solvent and may cause the organic solvents to evaporate. Undertake this step beneath a fume hood if necessary.