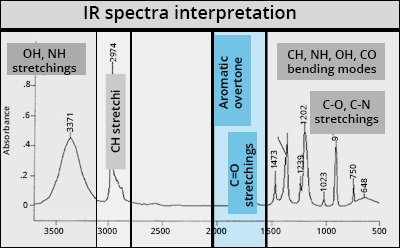
Basics of vibrational spectroscopy
C=O stretchings
Carbonyl function
Very intense and a little broad in the presence of hydrogen bonds
Most typical band: νC=O from 1800 to 1600 cm-1
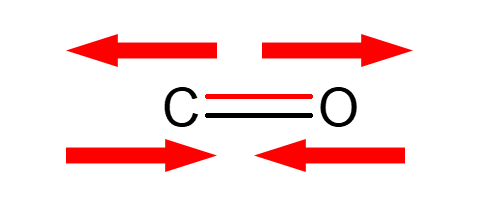

Strategy
- Ketones:
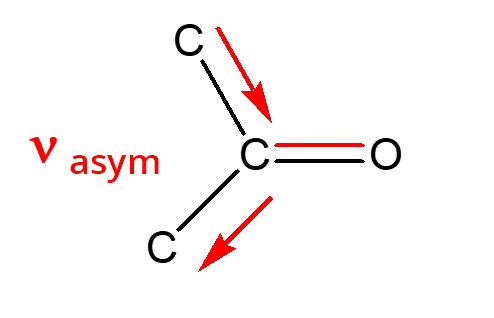
- Aldehydes:
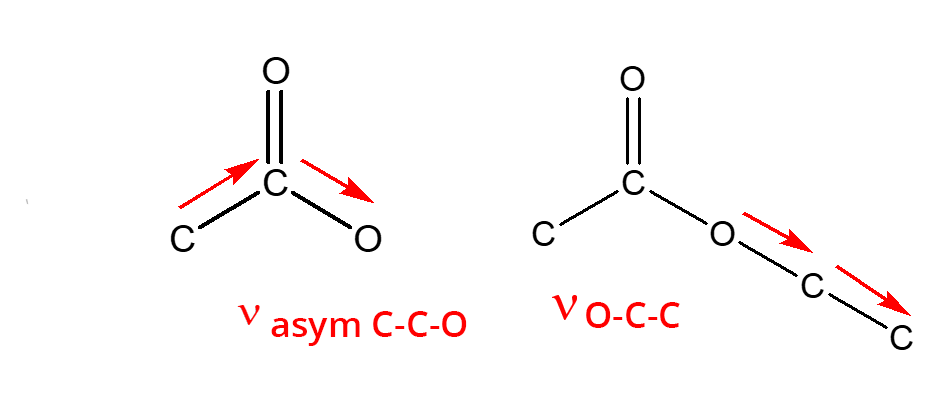
- Esters: 3 intense and rather broad bands
νC=O = 1700 cm-1
νC-C-O = 1200 cm-1
νO-C-C = 1100 cm-1
νC-C-O = 1200 cm-1
νO-C-C = 1100 cm-1
- Carboxylic acids:
- Carboxylates: 2 intense bands
νsym C=O = 1450-1360 cm-1