Basics of vibrational spectroscopy
Interpretation of IR spectra
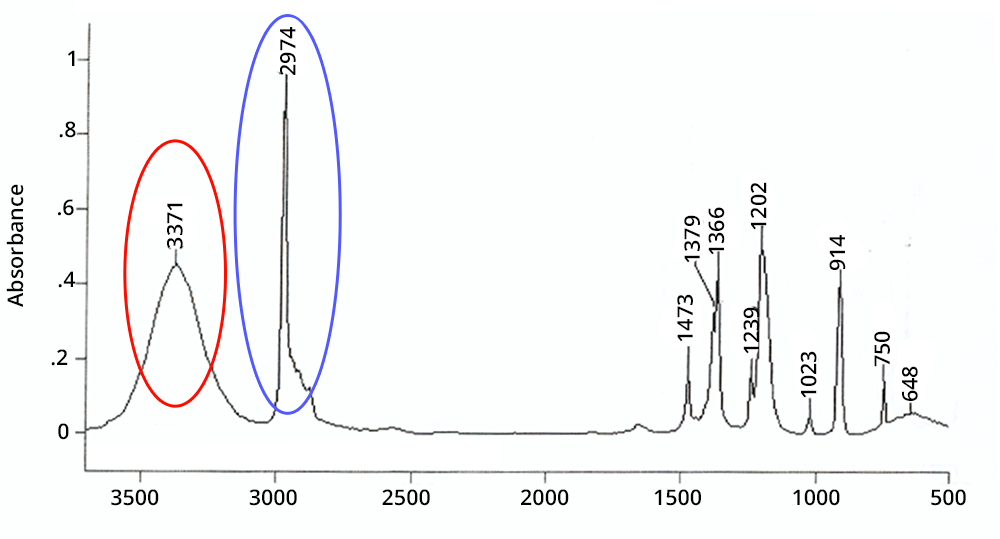
The first useful step in interpreting spectra is the analysis of their general appearance:
A peak is wide if it corresponds to a chemical function involved in hydrogen bonds or if it is a combination or an overtone - in the latter case its intensity is very weak.
A peak is intense in IR if the corresponding chemical function exists several times in the molecule (additivity of Beer-Lambert’s law) or if the atoms involved have a large difference in electronegativity (e.g. CO, OH).
Click on each circled area to find out more
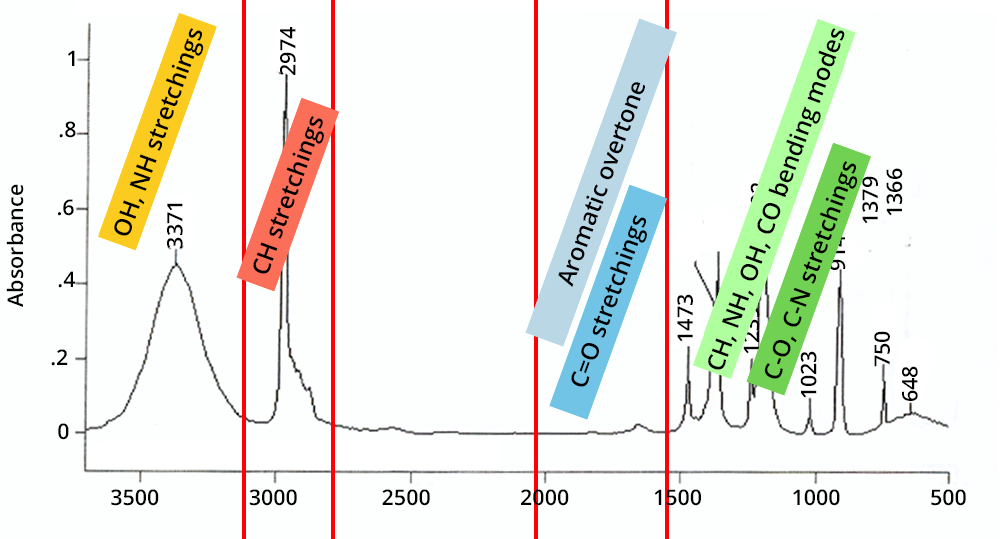
The second step is to compare the position of the vibrations with existing interpretation tables.
The position of the peaks depends on the strength of the bond between the two atoms and on their reduced mass:
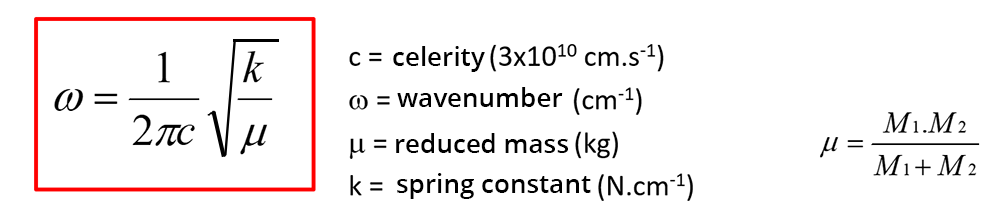
Strategy: start with high wave numbers and confirm the stretches by their associated deformations.
Click on each box to find out more