Basics of vibrational spectroscopy
Raman scattering
Raman spectroscopy or Raman scattering is a vibrational spectroscopy complementary to IR spectroscopy. It is based on the same physical phenomenon: bond vibrations between atoms of a molecule, which correspond to transitions allowed between different vibrational levels. This analytical technique is also non-destructive, does not require any sample preparation, and is adapted to any kind of sample, whether solid, liquid or gas.
Principle
Contrary to infrared, Raman spectroscopy is based on a scattering phenomenon. Any molecule excited by a light beam diffuses light in every direction. This scattering can be elastic, meaning that the scattered photon has the same frequency as the incident photon (Rayleigh scattering). The Raman effect occurs when the frequency of the scattered photon is different from that of the incident photon (Stokes and anti-Stokes scattering).
The energy difference between Rayleigh scattering and Stokes or anti-Stokes scattering corresponds to the vibrational energy of the molecule. The Raman spectrum is obtained by considering the difference in wave number between elastic and inelastic scattering.

An electric field slightly distorts the electron cloud of a chemical molecule. Polarizability (α) can be defined as the ease of distortion of the cloud.
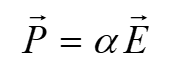
All molecules have polarizability. A molecule has Raman vibrations if the derivative of its polarizability with respect to x (coordinate) is non-zero.
Equipment
The different components of a Raman spectrometer are:
- an intense monochromatic light source: laser
- an interferometer
- a sample compartment
- a monochromator (to suppress stray light)
- a detector




