CHOOSING A WAVELENGTH
The measurement must be done when at a maximum of absorption. This is to prevent against any instrumental deviation (where the desired wavelength is not the wavelength used in practice by the spectrometer) or any spectrum modification (of which, strictly speaking, the shape depends of factors such as temperature, ionic force, nature of the solvent, and the nature of other present molecules …), the measurement error of absorbance A is minimised if . Referring back to the studied molecule’s spectrum is necessary in order to determine the wavelength to work with. Many information sources are useful to find out the studied element’s spectrum:
- Some spectrums are available in scientific literature, for example: http://webbook.nist.gov/chemistry/
- Many rules summarise the relations between molecular structure and their UV properties: mainly summed up by the rules of Woodward and Fieser.
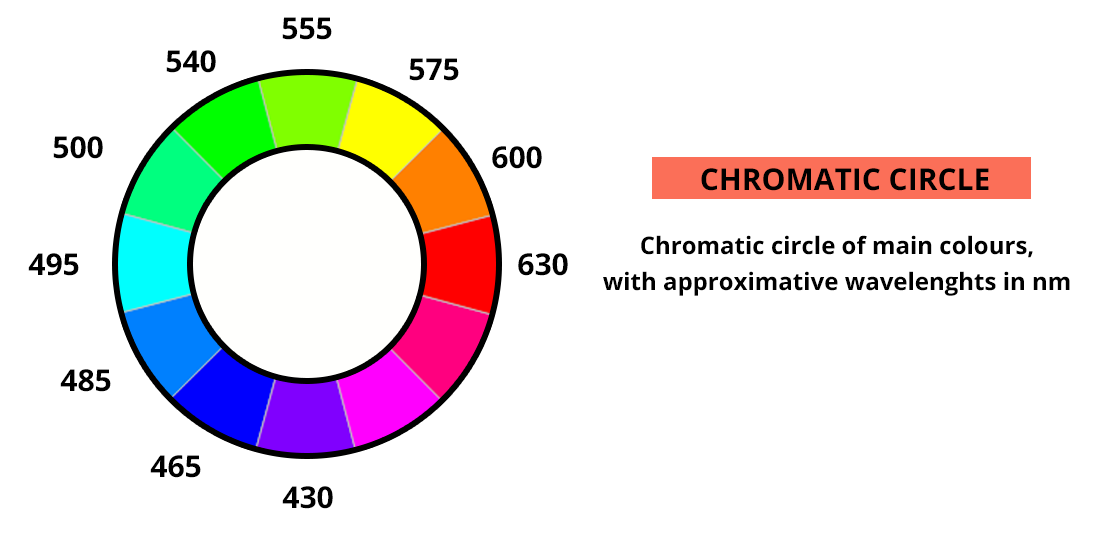
- In the visible spectrum, the colour of the compounds are a first indicator of their absorption wavelength: the solution appears coloured, meanings it absorbs beams of the complementary colour which is the colour diametrically opposed on the chromatic circle. In this way, the absorbance of a solution of potassium permanganate (magenta) will be measured at a wavelength of 530 nm.
In case of complete absence of information and/or to acquire a spectrum measured in controlled experimental conditions, one needs to establish oneself the spectrum by following the protocol detailed here.




