Basics of UV-visible absorption spectroscopy
The Woodward and Fieser rules
The empirical rules of Woodward-Fieser are aimed at predicting the wavelength of the maximum absorption in the UV-visible domain for organic compounds. They edict that some groups of functions based on their nature may induce or not a different increase of the wavelength for the maximum absorption: they are named chromophores. The wavelengths of maximum absorption for dienes (basic structures) as well as increments for several groups constitute the Woodward-Fieser rules. For polyenes, the Fieser-Kuhn rule is used instead.
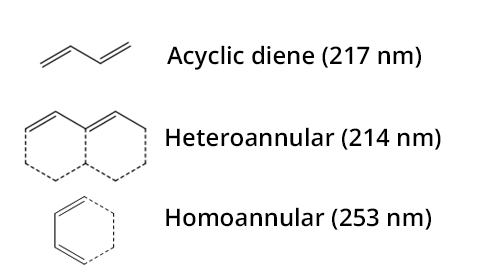
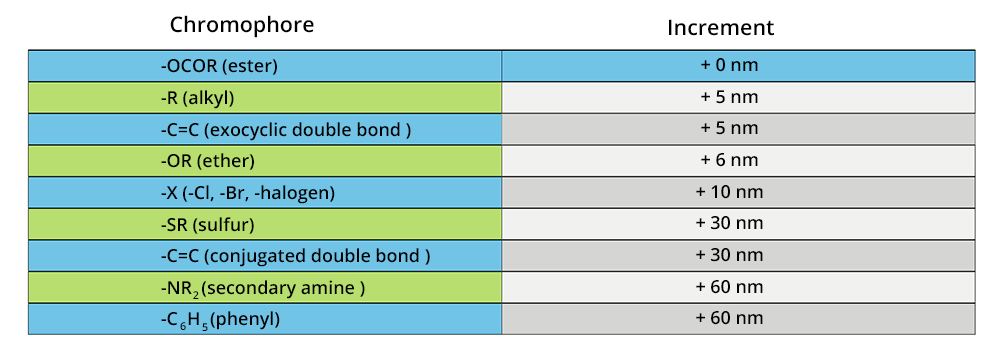
Fieser-Kuhn rule
λmax = 114 + 5M + n (48,0 - 1,7 n) - 16,5 Rendo - 10 Rexo
where
λmax is the wavelength of the maximum absorption
M is the number of alkyl substituents/ residues of cycle in the conjugated system
n is the number of conjugated double bonds
Rendo is the number of rings with endocyclic double bonds in the conjugated system
Rexo is the number of rings with exocyclic double bonds in the conjugated system
and εmax = (1,74 x 104) n where εmax is the maximum absorption coefficient.




