Basics of UV-visible absorption spectroscopy
Principle
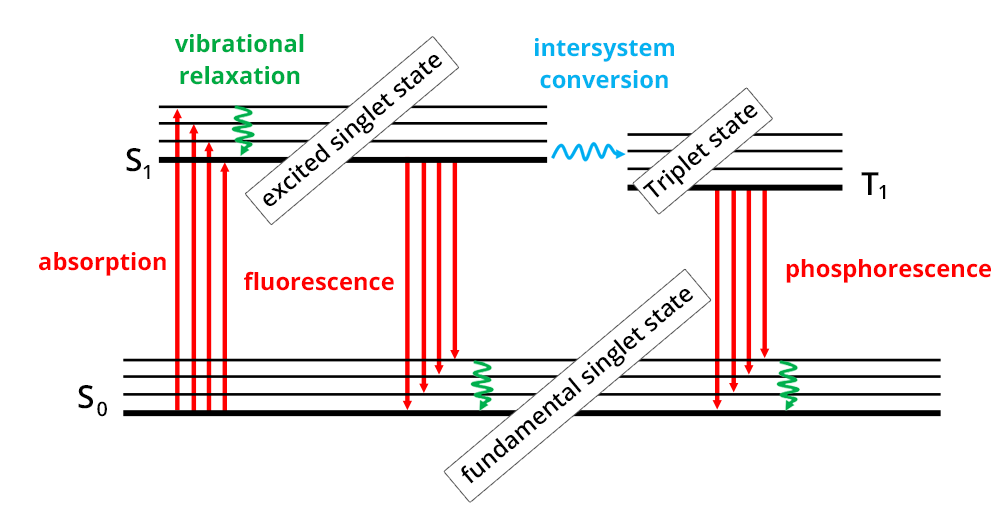
The absorption phenomenon is based on the interaction of electromagnetic radiation and electrons. When a molecule absorbs light, radiation induces electronic transitions, i.e. the transition of an electron from its fundamental to an excited electronic state (Perrin-Jablonski diagram).
The electromagnetic wave is characterized by its energy or its wavelength based on the following equation: E = hc/λ
with:
h = 6.62x10-34 J.s (Planck constant)
c = 3x108 m/s (speed of light)
λ = wavelength (in nm)
As electronic states of a molecule are quantified, absorption can occur if the following rule is confirmed: ΔE = Eexcited state – Efundamental state
It means that the energy of the light radiation is at least above or equal to the difference between both states, the fundamental and the excited one.
The energy of molecular orbitals being specific to each group or chemical function, absorption wavelength is thus correlated to the type of chemical bonds.