ON WHICH PRINCIPLE?
Dielectric forces that maintain the cohesion of the ion pair (AB) are given by Coulomb’s law:
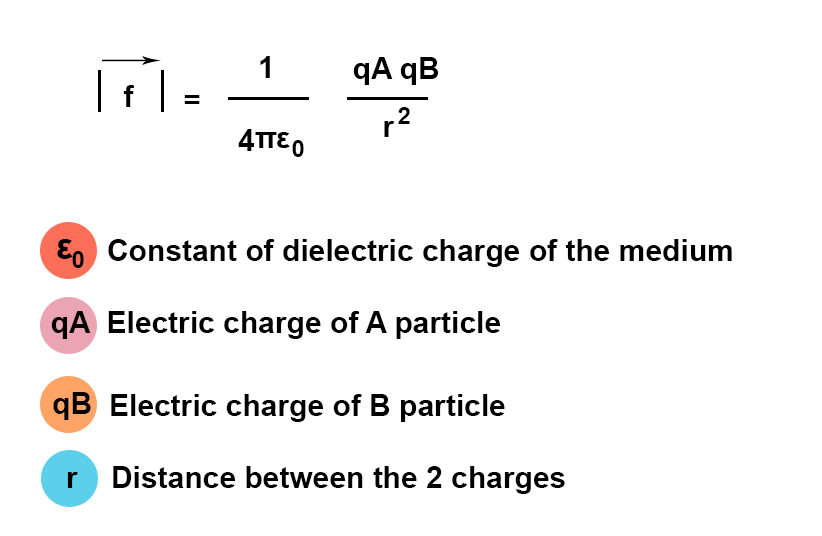
The notion of ion pair will be written in parentheses, for instance (A-B+)
Ion pairs are unlikely to be found in solvents that have a high dielectric constant such as water, and are therefore very dissociative.
This is reinforced by the fact that single electrolytes form ion pairs in water significantly only at concentrations above 1M.
Correspondingly, ion pairs are favored in organic solvents given their low ε0, and when the organic solvent content of hydro-organic mixtures (such as methanol or acetonitrile) is increased, for instance in liquid chromatography eluents.




