Potentiometric titration of base salts
POTENTIOMETRIC TITRATION OF LIDOCAINE HYDROCHLORIDE
Titration is carried out in the laboratory using:
- A tittered sodium hydroxide solution of concentration [c] = 0.0496 M made up according to the European pharmacopoeia standards
- A test sample of lidocaine hydrochloride: 72.5 mg raw material
- A theoretical expected volume of NaOH from the burette (assuming that the raw material is pure):
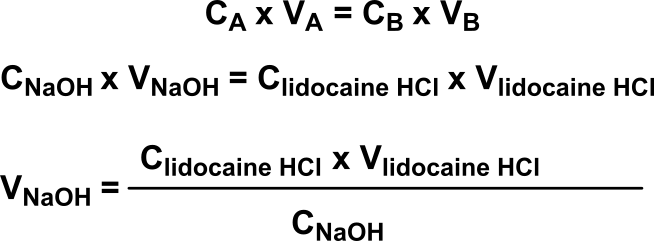 (1)
(1)N.A.: Letting nlidocaïne.HCL be the number of moles of raw material used in the titration, the following can be written:


Integration of this value in equation (1) gives:





