Choosing and developing an extraction method
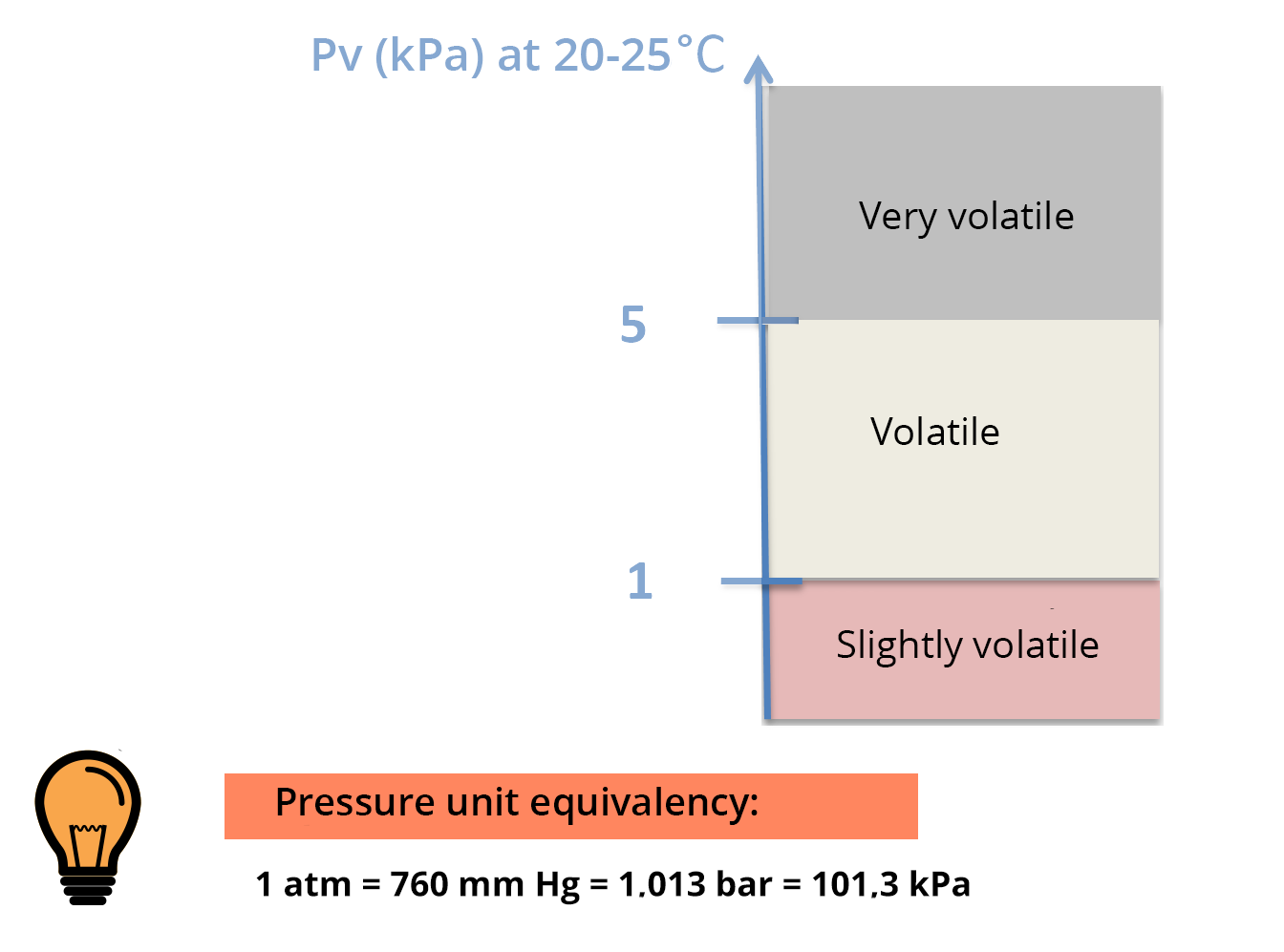
Saturated vapor pressure
Saturated vapor pressure is the pressure, at equilibrium, exerted by the volatilized solute molecules above a solution of pure solute.
The higher the volatility of the solute, the higher the saturated vapor pressure will be (for a given temperature) – see PV scale.