Validation
The validation of a method is “the confirmation by examination and the provision of objective evidence that the particular requirements for a specific intended use are fulfilled” (ISO/IEC 17025).
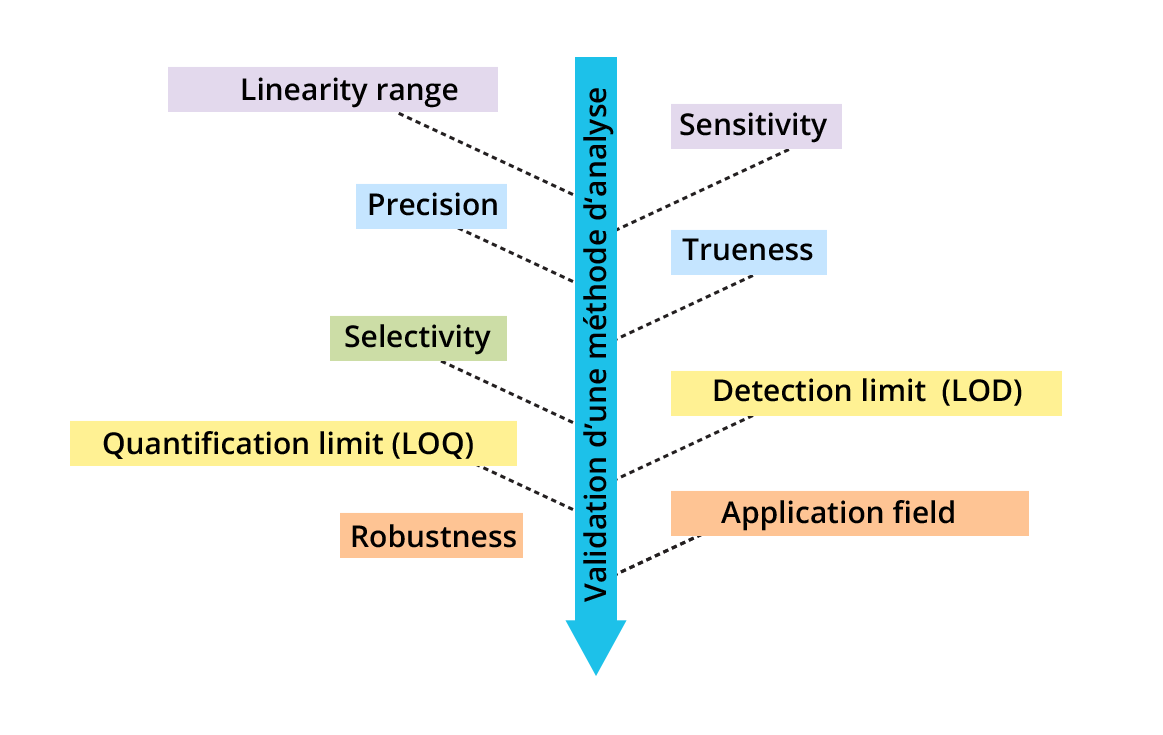
The validation of a method requires the evaluation of a certain number of performance characteristics of the analytical method (see figure). This is an intra-laboratory validation (i.e. validation within the laboratory). Ideally, this should be complemented by an inter-laboratory validation (validation during a trial involving several qualified laboratories).
Concentration range for which the calibration model is linear
Close agreement between the measurement results of the same measurand (depending on the conditions: repeatability, intermediate precision or reproducibility)
Capacity of the method to determine an analyte in a mixture (free from interference from other compounds)
Smallest concentration of the analyte that can be quantified with a known risk of error
Capacity of the method not to be affected (= no influence on the result) by small variations in certain parameters
Quotient of signal change and the corresponding concentration change (= slope of the calibration curve)
Close agreement between the mean of repeated measurement results and the expected reference value
Smallest concentration of the analyte that can be detected (but not quantified) with a known risk of error
Range of concentrations for which the method was validated (the application field can be smaller than the linearity range)
Click on each item to find out more

If a method standardized by an official body is used, this means that the method has already been validated. Nevertheless, the laboratory still needs to ensure that it is capable of applying this method – it must check that it is able to achieve the indicated method performance of the standard method.