Determination of Cl- ions by colorimetric titration: Charpentier-Volhardt’s method
Procedure
- Withdraw exactly 10 mL of the water to be analyzed and transfer with a pipette into a 100 mL Erlenmeyer flask.
- Pipette exactly 20 mL of silver nitrate solution at 0.1 mol/L. A white solid precipitates.
- Add 1 mL of a concentrated nitric acid solution and roughly 1mL of iron nitrate (III) at 1 mol/L. Carefully filter the solution by rinsing the precipitate with distilled water and adding the wash water to the filtrate.
- Titrate the filtrate with a solution of potassium thiocyanate at 0.10 mol/L.
- Stop dosing when there is a persistent red color
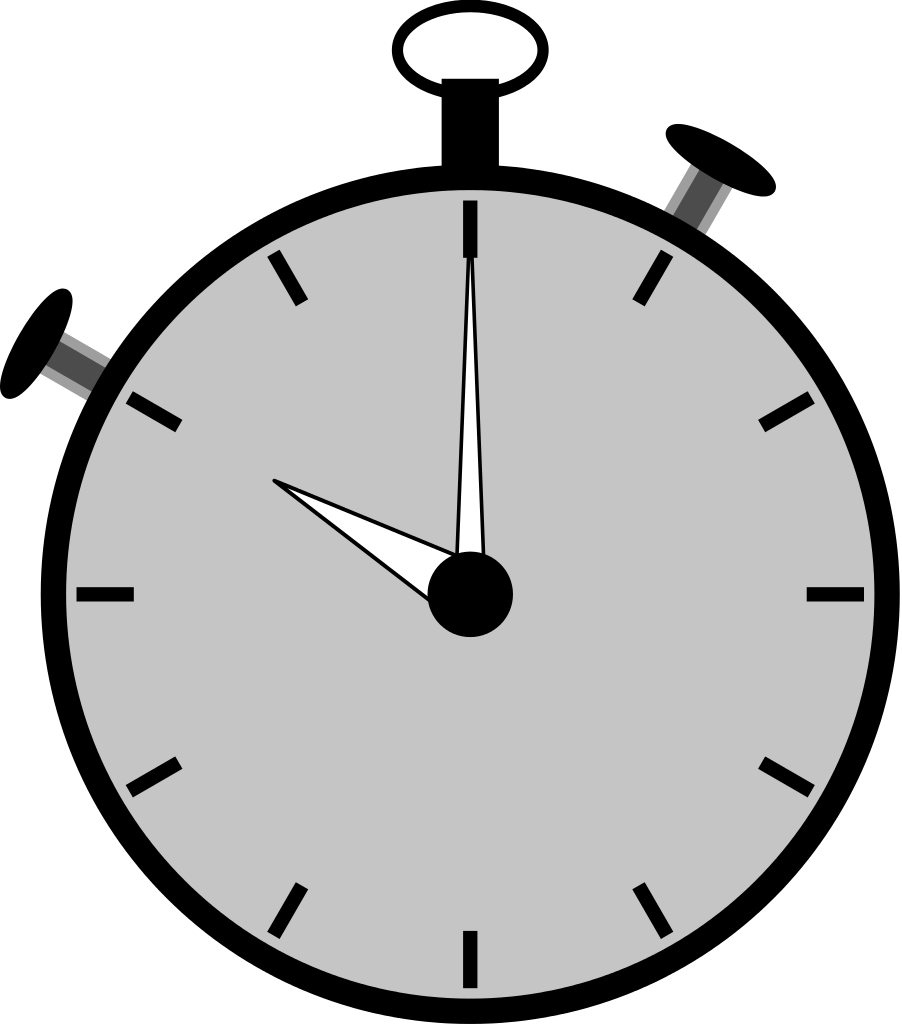 ≈ 30 minutes / sample
≈ 30 minutes / sample




