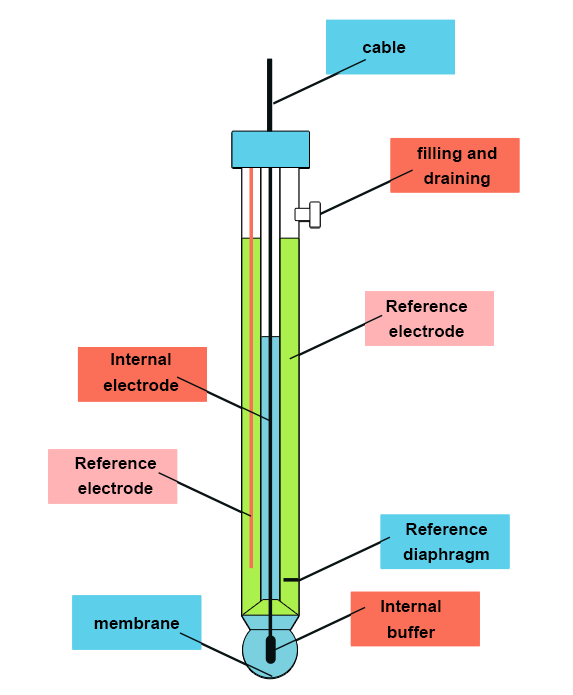
Determination of sodium ions using a selective electrode (potentiometry)
Constitution of the electrode
The electrode selective to Na+ ions is similar in its design to the standard glass electrode (selective to H+) but without an internal reference electrode. It needs to be combined with an external reference electrode (e.g. Ag/AgCl). However, the composition of the glass is different: here it is composed of 71% of SiO2, 18% of Al2O3 and 11% of Na2O. This composition makes it selective to Na+ ions.