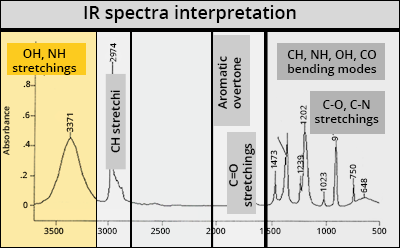
Basics of vibrational spectroscopy
OH or NH function?
Peaks above 3100 cm-1 correspond to OH or NH stretching modes. They are large in the presence of hydrogen bonds:
νO-H = 3350 ± 50 cm-1
νN-H = 3320 – 3280 cm-1 (for an alkyl)
νN-H = 3400 cm-1 (for an aromatic)
νN-H = 3320 – 3280 cm-1 (for an alkyl)
νN-H = 3400 cm-1 (for an aromatic)
To confirm the presence of a NH or OH function, their stretching mode must be correlated to bending vibrations.