Basics of UV-visible absorption spectroscopy
Absorption bands position
The position of an absorption band can be influenced by several factors such as solvent, steric, inductive or mesomeric effects …..
Hypsochromic shift
A hypsochromic shift is a shift towards lower wavelengths (UV)
Bathochromic shift
A bathochromic shift is a shift towards higher wavelengths (IR)
Example
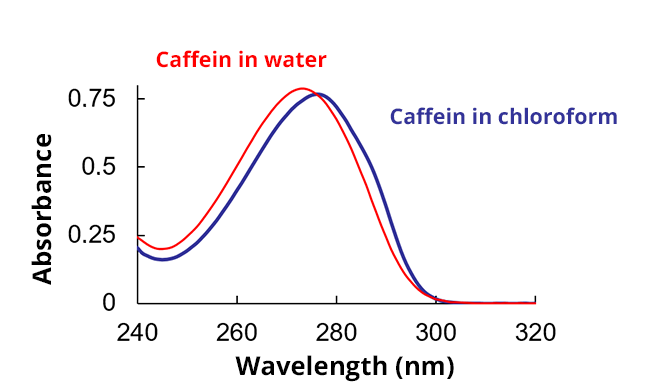
In chloroform, the absorbance of caffeine is upshifted (bathochromic shift) compared to its absorption in water. The energy difference between fundamental and excited states is smaller in chloroform.
Woodward-Fieser rules:
The Woodward and Fieser rules allow to predict the wavelength for the maximum of absorption of conjugated systems.
When the number of delocalized electrons increases in a molecule, its chromophores face a bathochromic effect.
Schematic representation
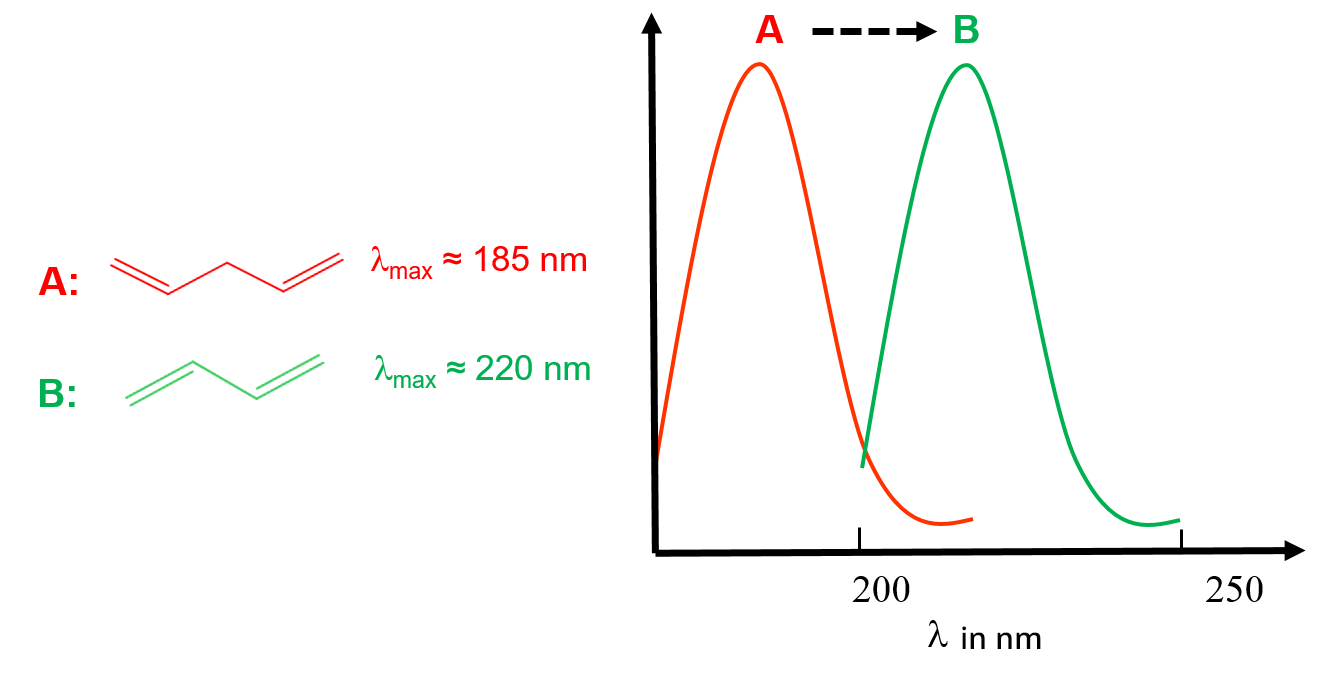
Molecule A possesses one C=C bond responsible for its absorption at 185 nm. Molecule B possesses the same chromophore but with delocalized electrons. Its absorption band is then shifted to higher wavelengths: bathochromic effect.
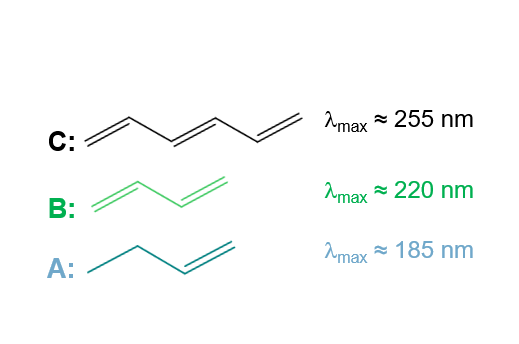
Molecules A, B and C possess the same chromophore, C=C, but with an increasing number of delocalized electrons. Their absorption is therefore upshifted (bathochromic effect).