Basics of UV-visible absorption spectroscopy
Absorption bands intensity
Absorption bands intensity is related to the electronic transition probability, with a molar absorption coefficient ε λmax which is valid for one wavelength.
10 ≤ ε ≤ 1000
1000 ≤ ε ≤ 100000
ε ≥ 100000
partly allowed transition
allowed transition
highly allowed transition
Absorption bands intensity is measured with the absorbance Aλ = log I0/I
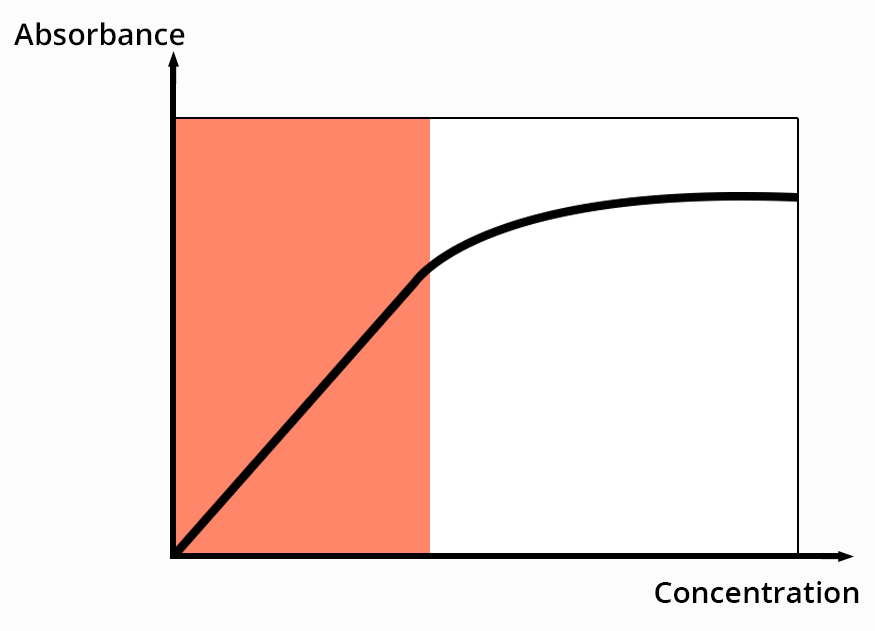
For a defined wavelength, the Beer-Lambert law links the absorbance to the concentration of species in solution.
with ελ the molar absorption coefficient (M-1.cm-1)
l the path length
c the concentration (mol.L-1)
The absorbance of a compound is proportional to the number of chromophores it possesses.
Additivity of the Beer-Lambert law
For a fixed wavelength, the absorbance of a mixture is the sum of the absorbances of each species.
As absorption bands position, their intensity can be modified by different factors (pH, solvent…).
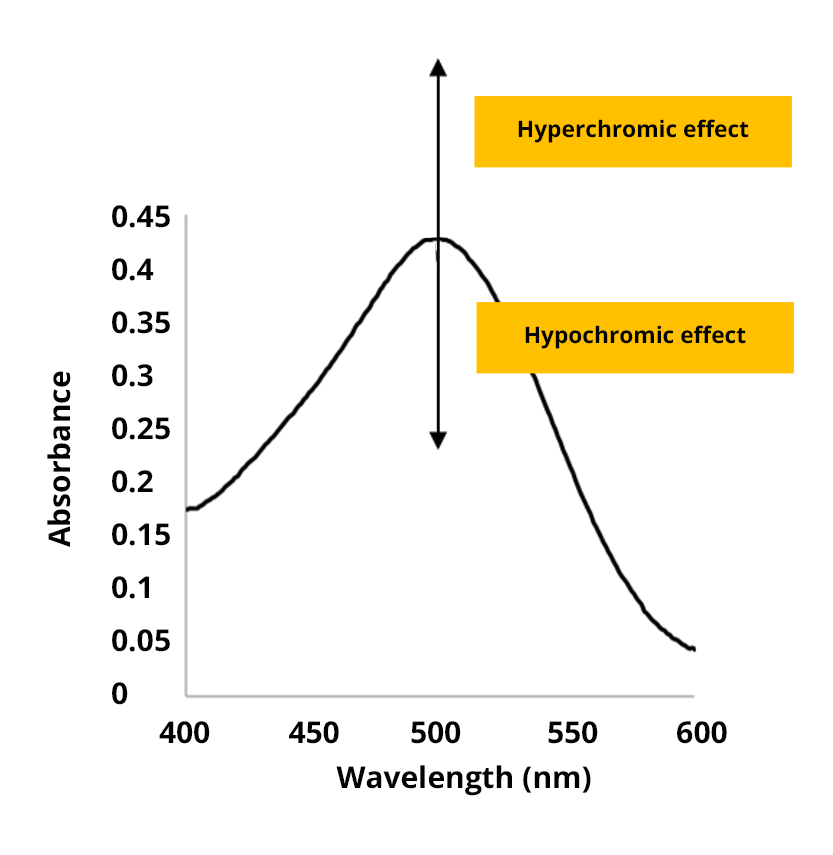
The hyperchromic effect
The hyperchromic effect corresponds to an increase in absorbance.
The hypochromic effect
The hypochromic effect corresponds to a decrease in absorbance.