A titration is used to determine the precise concentration, Cunknown (supposedly unknown) of a chemical compound, or analyte, in a solution, Eint.
During the titration, a chemical reaction occurs: therefore it is a destructive dosing method (the chemical we want to dose is modified by the reaction).
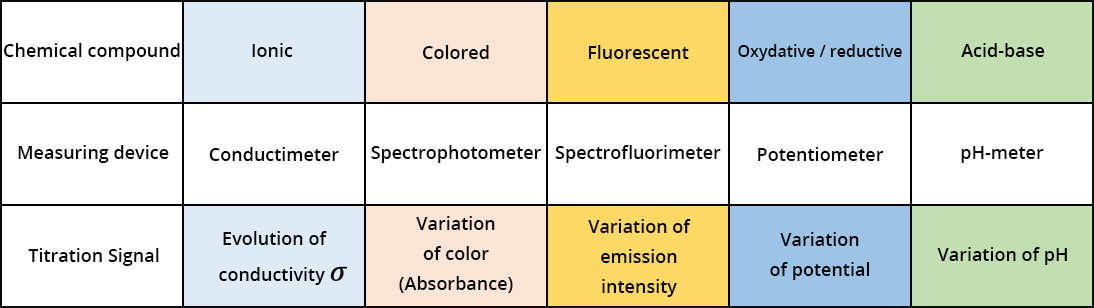
The nature and concentration of the different chemicals in the solution change during the titration. To determine Cunknown, we follow the variation of the signal S, given by the measuring instrument. The variation of the signal S depends on the volume of chemical reacting with the analyte we want to dose. A significant variation of the signal is observed when the last traces of the analyte are consumed.
Several measuring devices can be used (spectrophotometer, spectrofluorimeter, pH-meter, potentiometer, electrical conductivity meter, etc.) using the corresponding signals (absorption, fluorescence intensity, pH, potential, conductivity, etc.). In certain cases, Cunknown can be determined without the use of an instrument but instead by means of color differences between the analyte and one of the products formed by the reaction. A colored indicator can also be added to the solution, and this indicator is capable of changing color when there is significant variation in the signal (pH, potential, fluorescence intensity). This is called colorimetric titration. Only the latter type of titration method will be presented here.