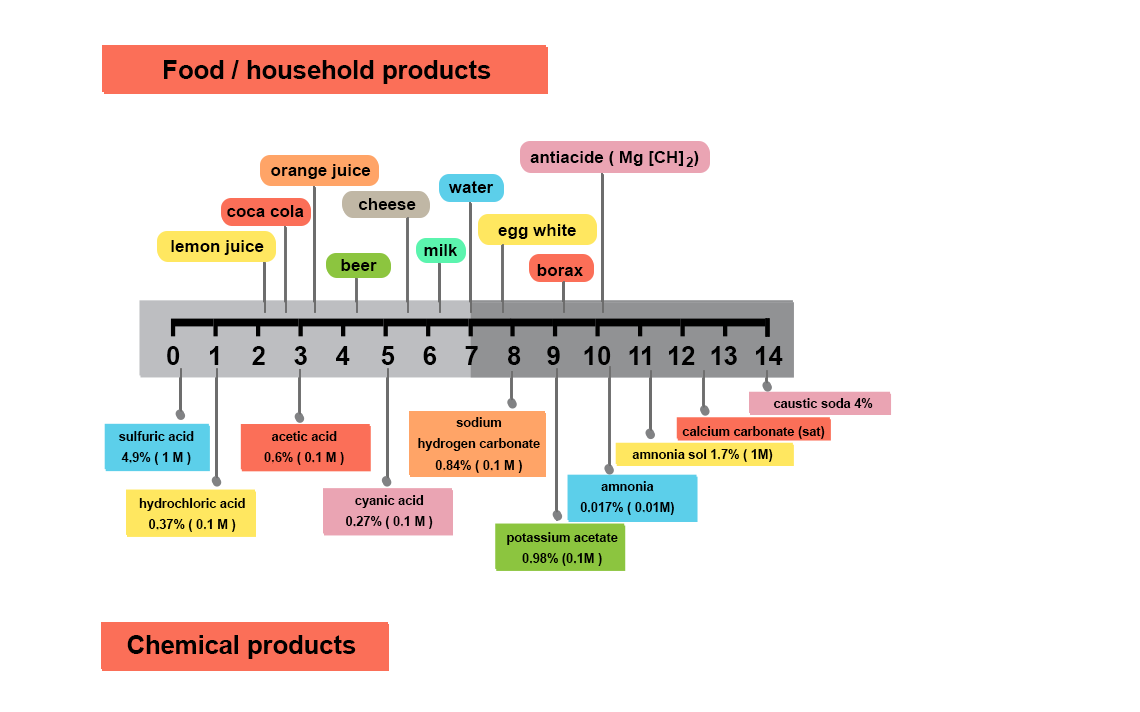
Why measure pH?
- To prepare a buffer solution at a controlled pH level necessary for carrying out chemical or biological experiments in optimal conditions.
- To monitor and direct a chemical reaction and/or the transformation process of food.
What is pH?
According to Sørensen, pH is defined as the negative logarithm of the concentration of hydronium ions, H3O+, resulting from the dissociation of molecules:
pH = –log [H3O+]
What is pH neutral?
Not only acids and bases dissociate to form hydronium or hydroxyl ions, pure water also dissociates to form these ions:
2 H2O ⇔ H3O+ + OH–
Kw = [H3O+][OH–] = 10–14 mol/L (25 ºC)
This is the case when concentrations of [H3O+] and [OH–] are 10–7 mol/L, i.e. pH 7.

The orders of magnitude of pH