Determination of hydrogen carbonate ions by pH-metric and conductimetric titration
Dosage principle
We conduct a colorimetric titration of 100 mL of water to be analyzed with hydrochloric acid at 0.02 mol/L. This titration can be monitored by both conductimetric and pH-metric methods.
Refer to the conductivity data sheetRefer to the pH-meter data sheet
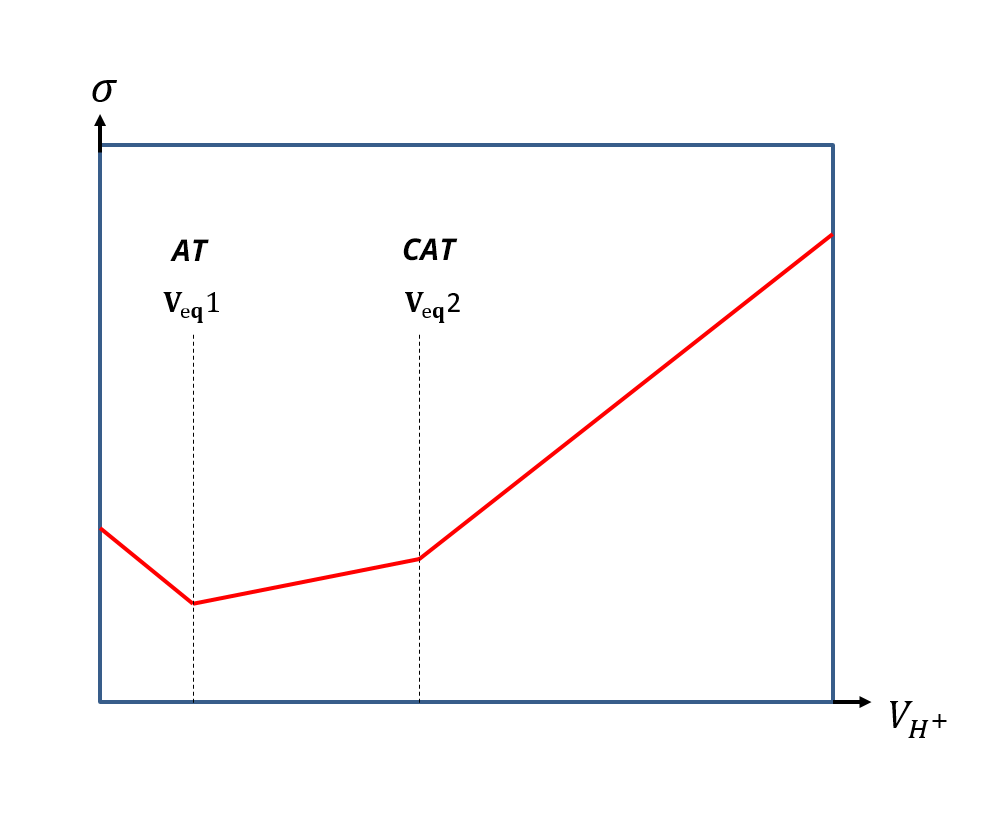
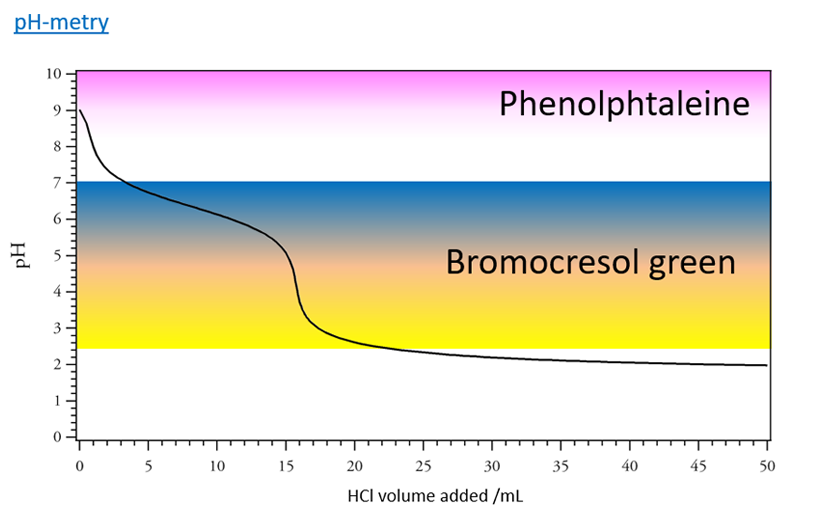
The AT is the volume of hydrochloric acid (expressed in mL) at a concentration of 2.0x10-2 mol/L required to dose 100 mL of water with phenolphthalein as the color indicator:
HO-(aq) + H+(aq) = H2O(l)
CO32-(aq) + H+(aq) = HCO3-(aq) + H2O(l)
CO32-(aq) + H+(aq) = HCO3-(aq) + H2O(l)
The CAT is the volume (in mL) of hydrochloric acid at a concentration of 2.0x10-2 mol/L equired to dose 100 mL of water with bromocresol green (or methyl orange) as the color indicator.
HO-(aq) + H+(aq) = H2O(l)
CO32-(aq) + H+(aq) = HCO3-(aq) + H2O(l)
HCO3-(aq) + H+(aq) = CO2(aq) + H2O(l)
CO32-(aq) + H+(aq) = HCO3-(aq) + H2O(l)
HCO3-(aq) + H+(aq) = CO2(aq) + H2O(l)
Data:
pKa (CO2,H2O / HCO3-) = 6.35; pKa (HCO3- / CO32-) = 10.35; pKa (methyl orange) = 3.4; pKa (bromocresol green) = 4.9; pKa (phenolphthalein) = 9.4; 𝜆°(HCO3-) = 4.45; 𝜆°(CO32-) = 13.9; 𝜆°(HO-) = 19.8; 𝜆°(H+) = 35.0; 𝜆°(Cl-) = 7.63