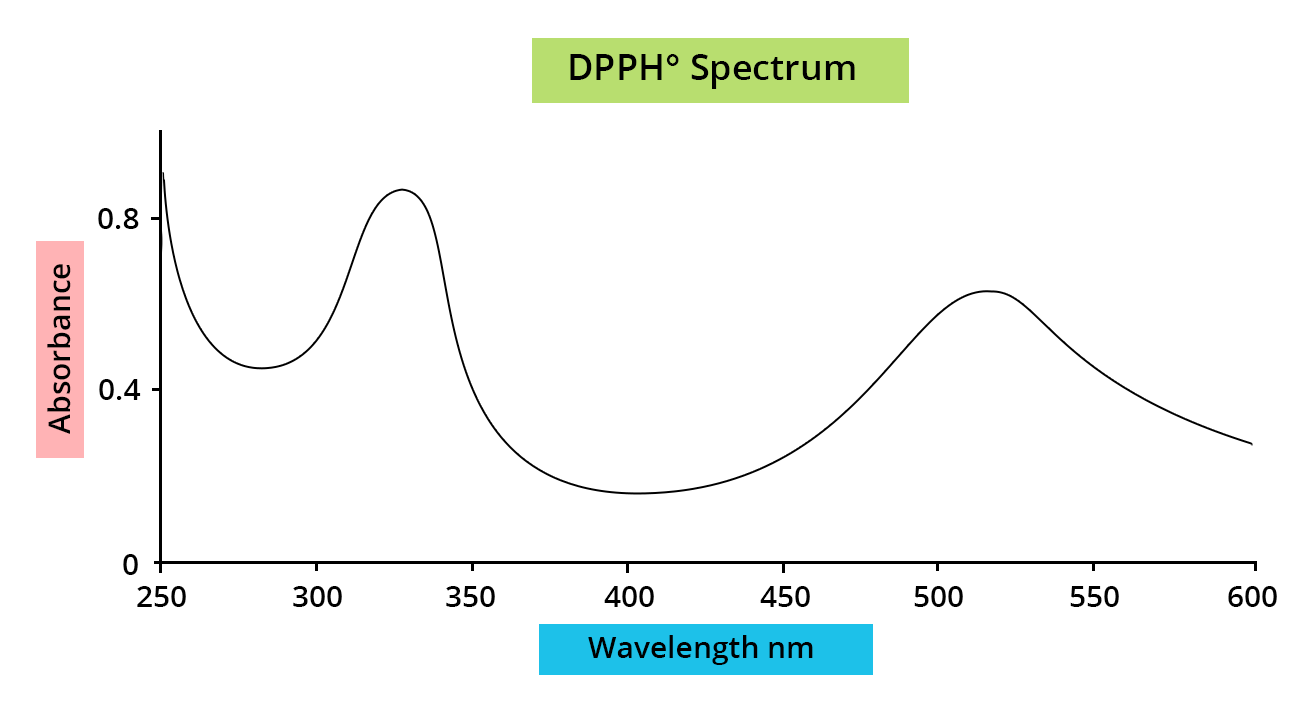
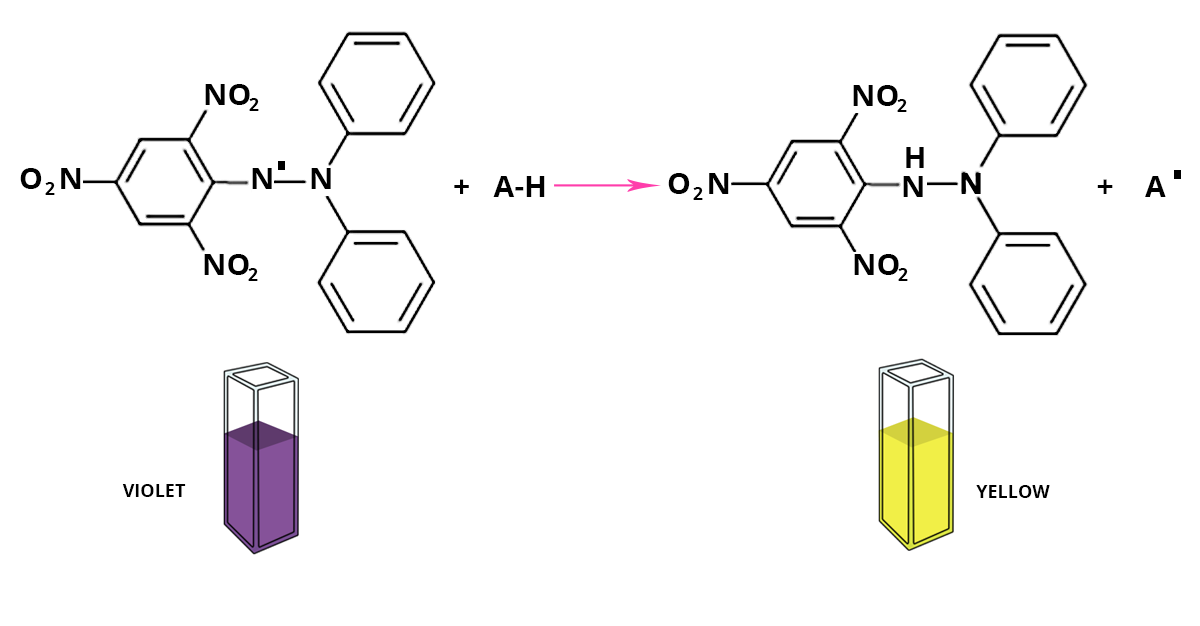
The DPPH° test is used to measure the antiradical power of pure molecules or plant extracts in a model system (organic solvent, room temperature). It measures the capacity of an antioxidant (AH, generally phenolic compounds) to reduce the chemical radical DPPH° (2,2-diphenyl-1-picrylhydrazyl) by hydrogen transfer. DPPH°, which is initially violet, becomes DPPH-H, pale yellow.
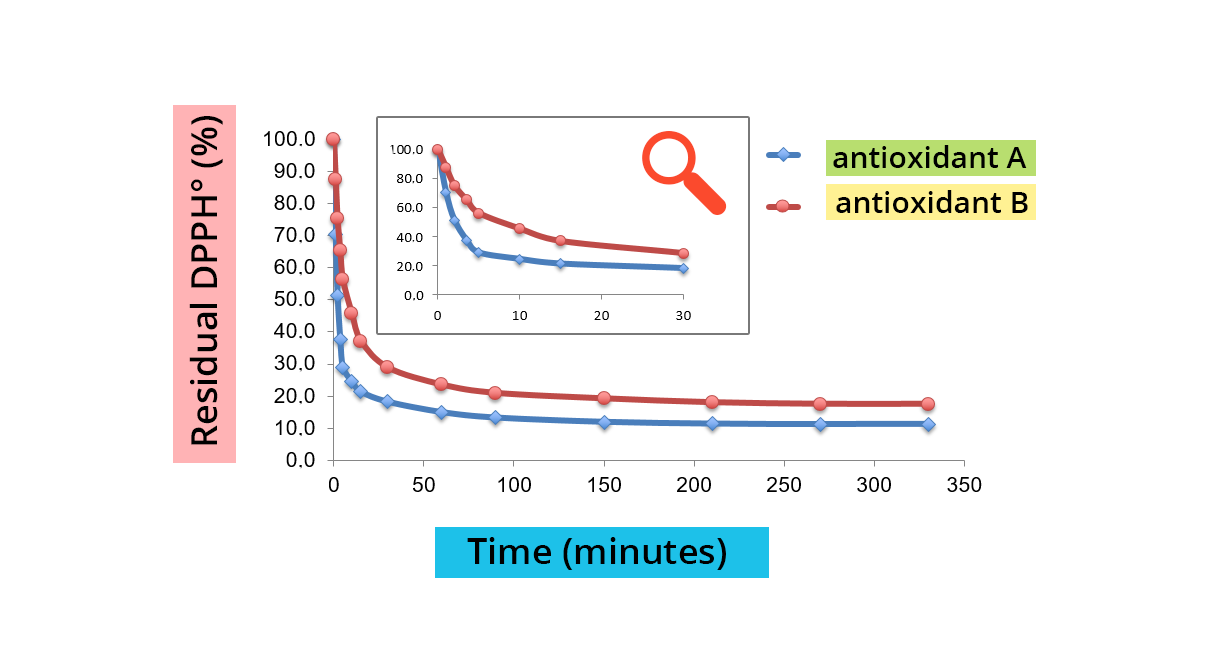
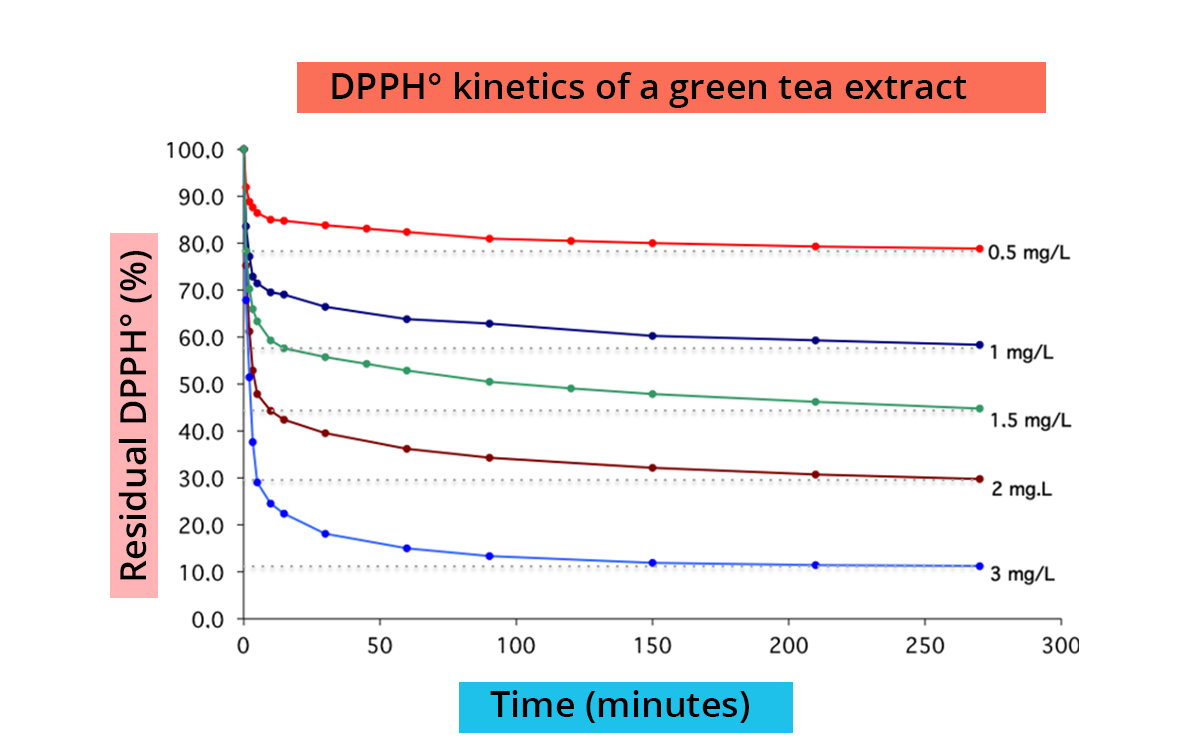
DHHPº reduction is easily measured by spectrophotometry at 515 nm (λmax DPPH°). The reaction speed depends on the nature of the antioxidant, and the amount of DPPH-H formed depends on the concentration of the antioxidant.