How do antioxidants act?
1. Primary antioxidants, also called FREE RADICAL SCAVENGERS,
are molecules capable of blocking lipid radicals (L°, LO°, LOO°) by hydrogen transfer. Example: LOO° + AH ⇒ LOOH + A°… This group consists nearly exclusively of phenolic compounds: synthetic antioxidants (BHA, BHT, TBHQ, gallates), tocopherols and phenolic compounds from plants (flavonoids, phenolic acids, diterpenoids…).
2. Secondary antioxidants, or PREVENTIVE ANTIOXIDANTS, act on the factors that promote oxidation: they chelate metallic ions (EDTA, citric acid, certain phenolic compounds), reduce oxygen (ascorbic acid), deactivate singlet oxygen (β-carotene)…
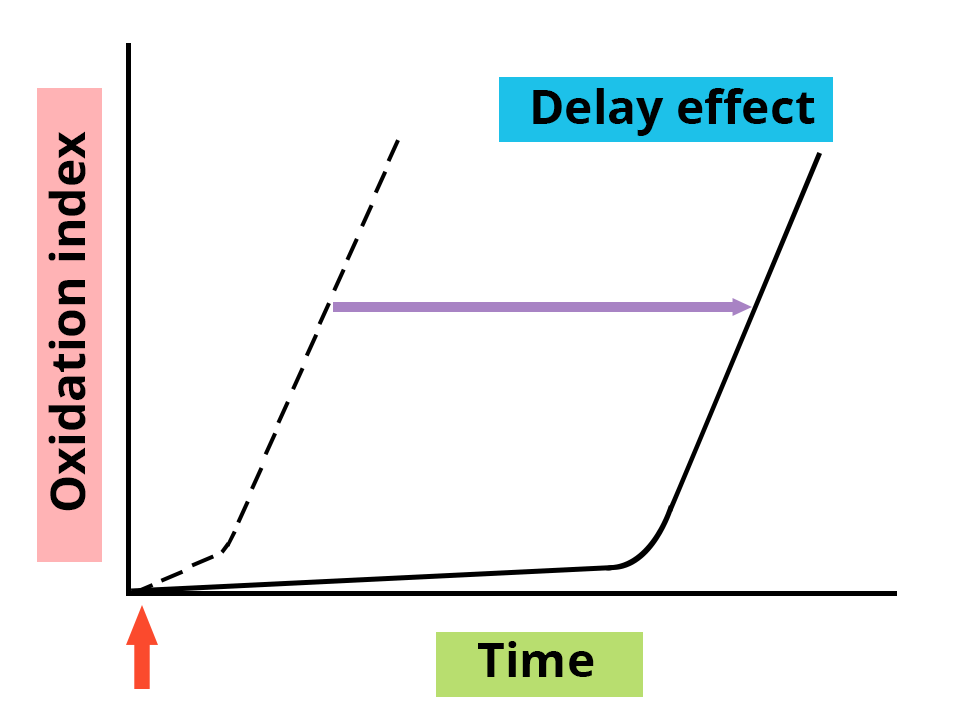
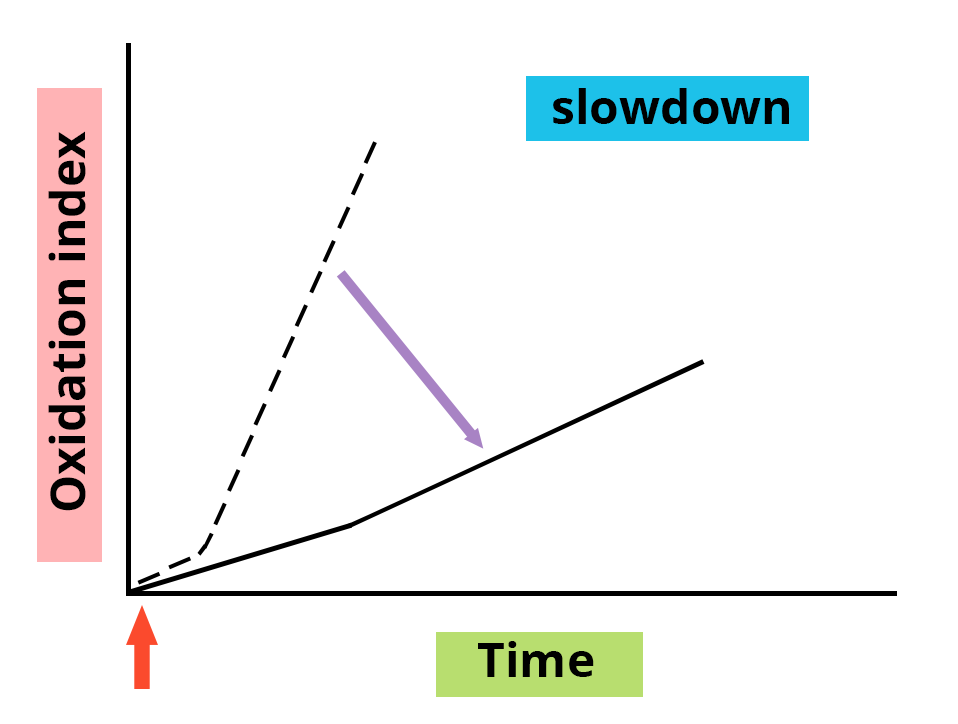
The presence or addition of an antioxidant is characterized by a delay effect or a slowing down of the onset of oxidation, an effect which varies depending on the nature of the antioxidant and its concentration.