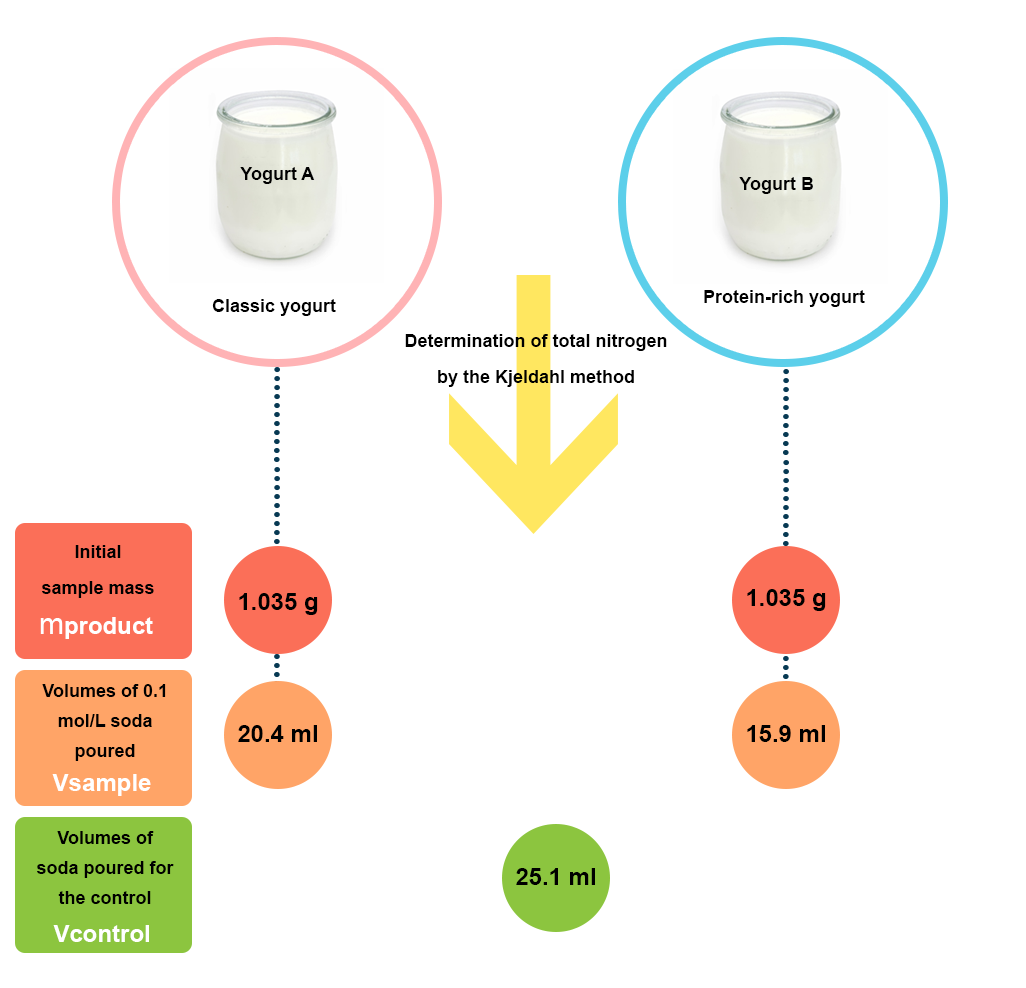
An example for practicing
You have to blindly control two yogurts. You know that one of them is a classic yogurt, while the other is a protein-rich yogurt. To this end, you carry out a determination of total nitrogen for each of the products (A and B) by the Kjeldahl method (see experimental procedure).
- the initial test sample mass (mproduct) is 1.035 g for each yogurt.
- the titration volumes of NaOH 0.1 mol/L (Vsample) are 20.4 mL for yogurt A and 15.9 mL for yogurt B.
- the titration volume of NaOH for the control (Vcontrol) is 25.1 mL.