How to perform a TLC?
Mechanisms involved
The compound to be analyzed is subject to a competition between:
- its adsorption on the stationary phase
- its solubilization in the eluent
- the adsorption of the eluent on the stationary phase
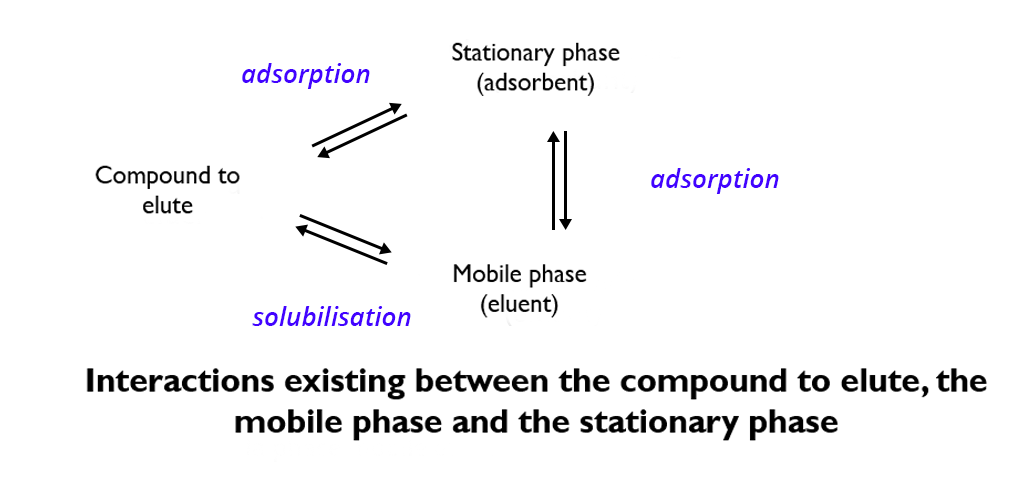
Two types of forces are involved when the eluent drives the solutes by adsorption chromatography:
⇒ Dissolution: solutes tend to dissolve in the eluent and migrate with it.
In this case, it has to be effective enough to outweigh the adsorbent’s adsorption capacity.
⇒ Displacement phenomena: eluent molecules look for the same adsorption sites as solute molecules and thus displace them.