Why saturate the elution chamber?
To achieve a reproducible TLC, the chamber must first be saturated with the vapor of the chosen eluent.
Indeed, it can be considered that the execution of a TLC actually involves three phases: the mobile phase, the stationary phase, and also the atmosphere (air) present around the TLC in the chamber.
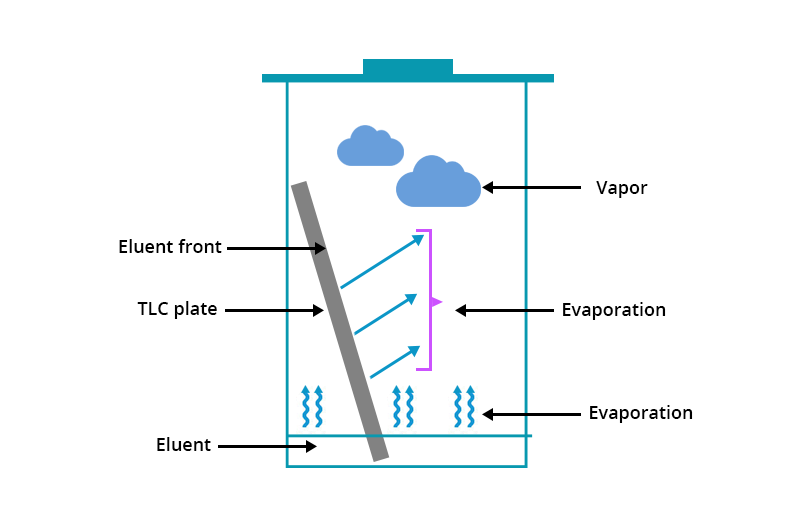
Migration of the eluent on the TLC plate is accompanied by its partial evaporation into the chamber’s atmosphere. The prior saturation of the latter ensures that it is at equilibrium and that the eluent velocity is established and reproducible. The determination of the Rf of the sampled substance will then be established and reproducible.
On the contrary, if the chamber is not saturated beforehand, the elution rate of the eluent is slowed down and varies according to the nature of the atmosphere in the chamber. Then the Rf value of the sampled substance will not be reproducible.