Origin of chemical shifts
The resonance frequency of the protons of a molecule will depend on the B0 field used for the NMR experiment since: ω0 = γ.B0. Thus, if a B0 field of 9.4 T is used, then the resonance frequency of the protons will be around 400 MHz.
On a NMR spectrum it is possible to distinguish different resonance frequencies. This difference originates from the environment close to the protons that resonate and in particular the screen constant σ that depends on the electrons close to the nuclei. Thus, depending on their environment, the protons will not be strictly subjected to the same B0 but to a specific and local B0 (B0 (local)): B0 (local) = B0 (1 – σ)
Hence, the inductive effects (donor + I and attractor-I) and the mesomeric effects (donor + M and attractor – M) - two electronic effects that are expressed in organic compounds according to the atoms present in the structure and their arrangement in chemical function - have a significant impact on the proton resonance frequencies of a compound.
Specific case of protons or groups of non-equivalent protons
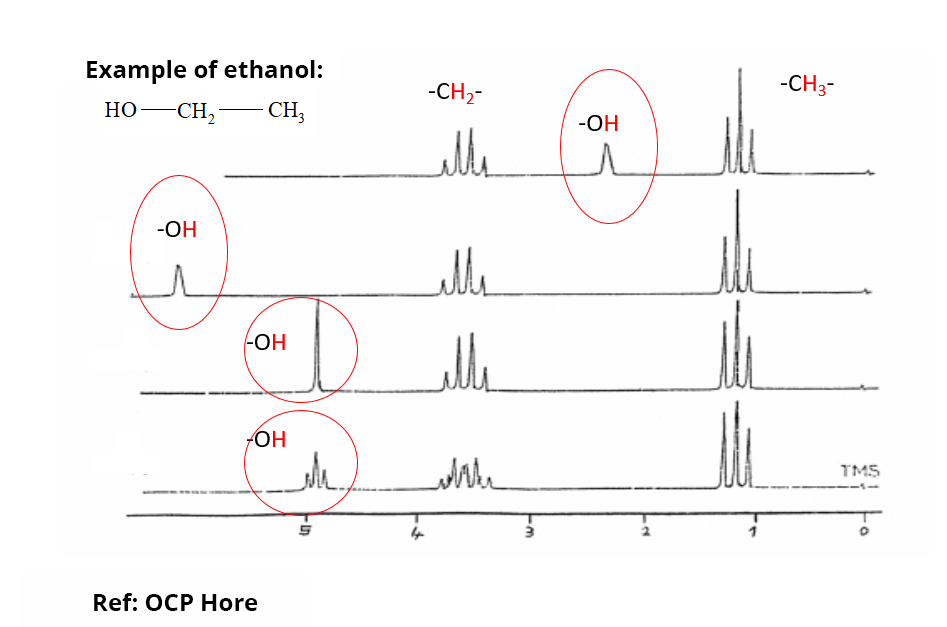
In practice, to express the resonance frequency of the nuclei of interest without being restrained by the value of the field B0, we use the chemical shift δ (ppm). The chemical shift variation of the different protons classically encountered in organic compounds is 20 ppm maximum. There are compilations of experimental chemical shifts for different nuclei that greatly assist in the analysis and interpretation of NMR spectra.
Chemical shift zones are characteristic of hydrogen atoms involved in particular chemical functions or environments (aliphatic or aromatic regions, etc.). Only labile protons can have a complete variation of their chemical shift depending on the sampling conditions.