Spectrum analysis strategy
In order to determine the structural formula of a compound, the study of the NMR spectrum of the proton (or others) requires a precise analytical methodology to propose a structure in line with the studied spectrum(s).
Stage 1: This step is to calculate the degree of unsaturation (DU) from the total raw formula of the compound.
Let’s take the raw formula: CmHnOpNqXr with X, a halogen.
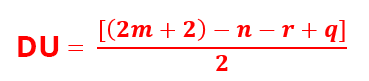
Stage 2: Determine the different chemical shifts (δ (ppm)) for the different signals. At this stage it is not possible to determine the exact chemical shifts because we do not yet have the multiplicity of signals. On the other hand, with integration, it is possible to know the number of protons that resonate under each signal identified.
In order to properly organize all the information that will be exploited, it is best to draw up a table.
Stage 3: For each signal we must then identify their multiplicity.
Stage 4: Once the multiplicity is known, the coupling constants (J (Hz)) can be measured.
To achieve stages 3 and 4, it is advisable to start by studying the simplest signals. Indeed, once a coupling constant is known unambiguously then it will be found on another signal since the phenomenon of spin-spin coupling is reciprocal. Steps 3 and 4 are very often carried out in parallel.

Stage 5: From the coupling constants of the table, we must identify the spin systems. A spin system is a set of nuclei that are coupled together but not to the other nuclei of the molecule. This step is very important because, from a spin system, it is possible to determine a fragment of the molecule, in accordance with:
- The multiplicity that gives the number of neighbors,
- The values of the coupling constants,
- The chemical shifts.
Stage 6: Once the structure of the fragments is established, we can propose a formula developed in accordance with:
- The chemical shifts,
- The raw formula,
- The degree of unsaturation.
Stage 7: Verification step. Starting from the proposed structure, we must verify that it corresponds in all aspects to the spectrum!