How to perform a titration
Titration principles
The titrant is placed in a graduated precision burette over a beaker containing a precise volume Vtitrand of the titrand solute whose concentration Ctitrand is to be determined.
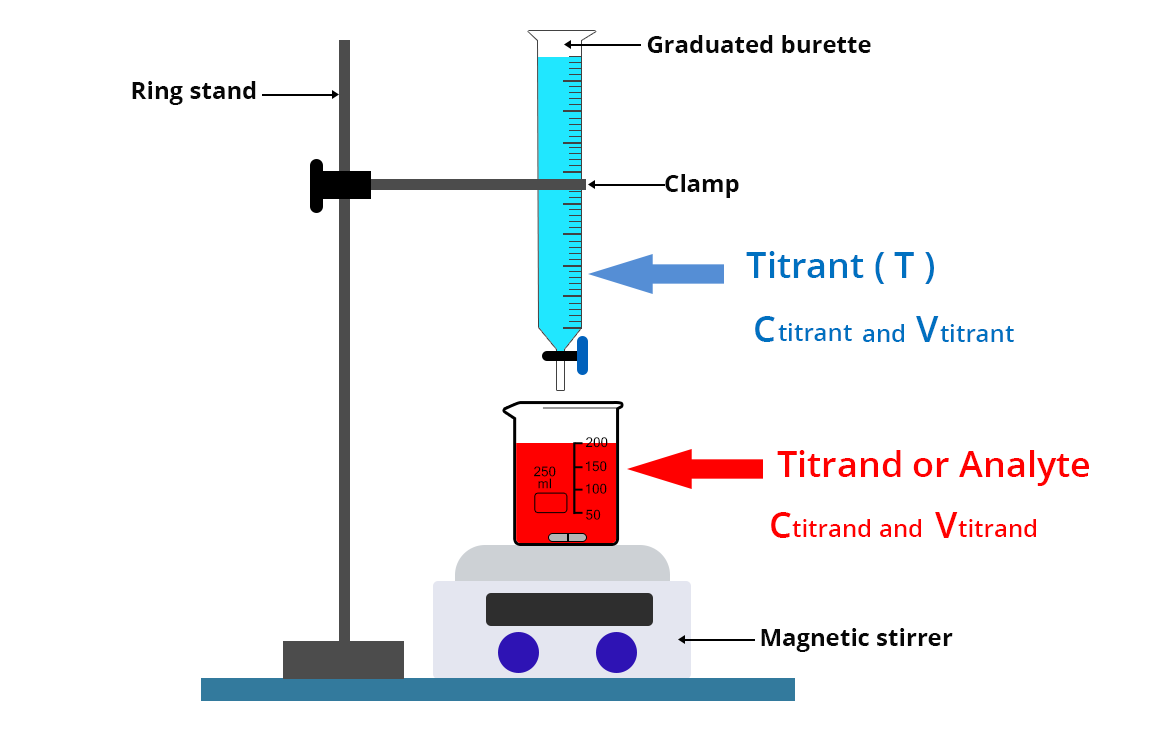
In the case of colorimetric titration, an Erlenmeyer flask can also be used instead of a beaker to avoid splashing. On the other hand, when this colorimetric titration is accompanied by pH-metric, conductimetric or potentiometric monitoring, it is necessary to take a beaker so that the electrodes and/or the conductimetric cell can be used.
Preparation
- Wash the graduated burette with distilled water then run the titrant solution through the burette once.
- Then place the titrant solution in the burette
- Collect a volume Vtitrand of the solution to be analyzed with a volumetric pipette and place it in a beaker rinsed beforehand with the titrating solution and dried. This sample is sometimes called the test sample.
- Add a magnetic stir bar and place the beaker on a magnetic stirrer.
- Carry out the titration.
Execution
When the experimenter tries to find the concentration Ctitrand , of the solution of interest, it is advisable to carry out a minimum of three titrations:
- The first one to find the end point of the titration (quickly carried out without waiting too long for the measurements to stabilize);
- A second one to obtain a first value of the titration point (Véq1) taking into account the uncertainties of the measurement. In practice the points must be measured more closely 1 to 2 mL before the equivalence point, until 1 to 2 mL after;
- A third one to assess the validity of the value found the second time and avoid any random errors. The value of the end point of the titration is noted as Véq2.
It must be verified that |Veq1 - Veq2| eq1 and Veq2 are the equivalent volumes of the two dosages and ∆V the uncertainty associated with the burette.
Finally: Veq = [(Veq1 + Veq2) / 2] ± ΔV




