Equivalence point
At equivalence the reagents are in stoichiometric proportions:

The unknown concentration Ctitrand is obtained through the volume Vtitrant called equivalence (or stoichiometric) point and often written as Véq.


If a reaction has a final extent rate τfinal below 0.95 it is not a successful titration because it is not quantitative. Therefore, the equivalence point cannot be determined precisely enough.
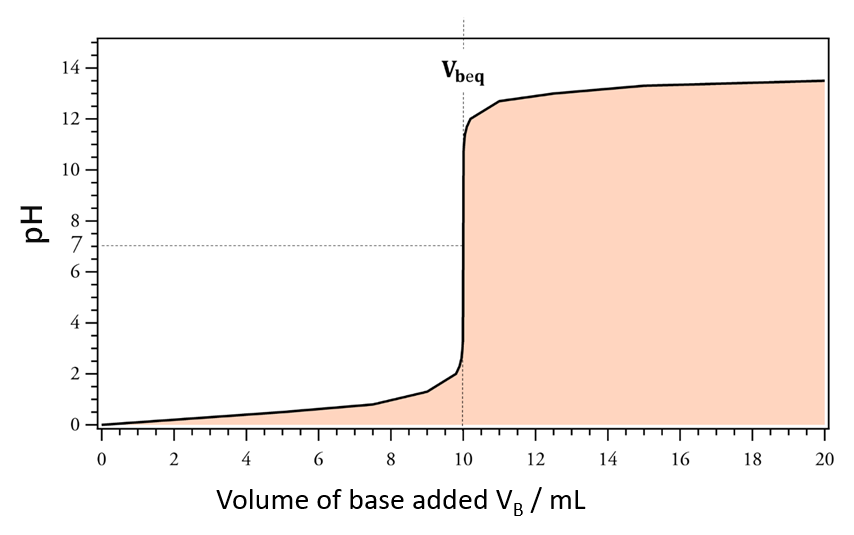
The end point of a titration is the point at which a physical modification occurs (change of color in a colorimetric titration, pH jump in pH-metrics jump in potential in potentiometrics, or a change of incline in conductimetrics) It is associated with the chemical equivalence condition (obtained theoretically).

The equivalence point is a theoretical value while the titration end point is an experimental value. There is a difference between the equivalence point and the titration end point due to the mismatch between the chemical modifications and our ability to observe them.