Redox titration
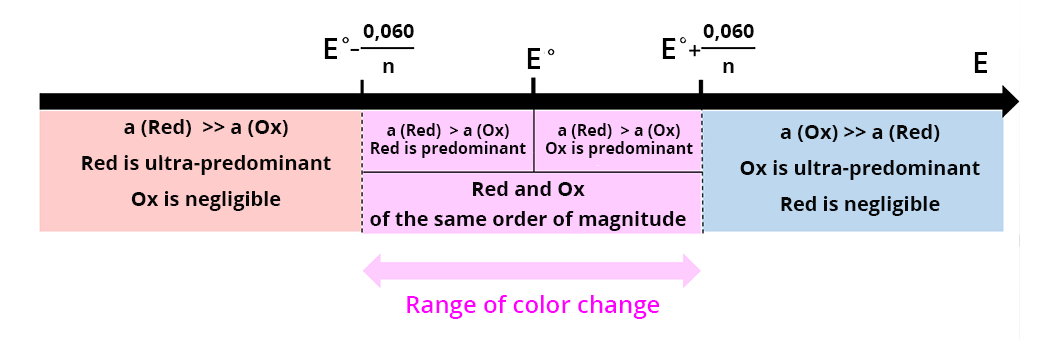
A redox indicator is a chemical species whose oxidized and reduced forms have different colors. The solution changes color in the vicinity of the standard potential of the couple. To avoid titrating the indicator, do not add too much of it.
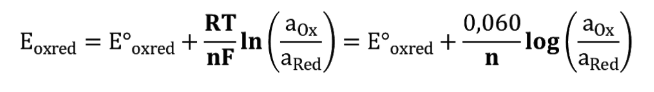
The transition range for this type of indicator is between 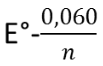 and
and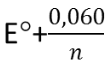
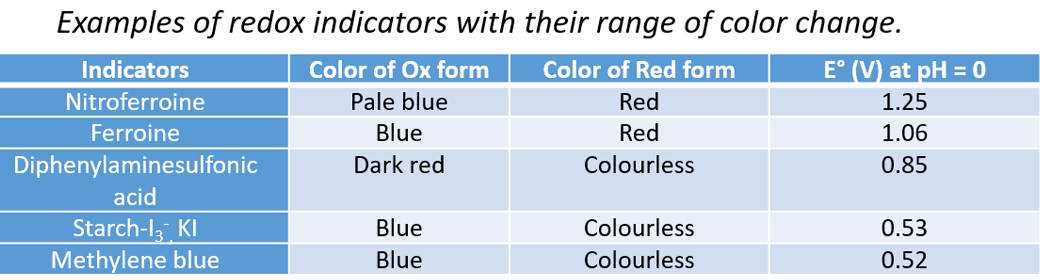

To precisely determine the end point, the potential E at the equivalence point must be within the transition range of the colored indicator.

In certain cases, the oxidized and reduced forms of the analyte of interest have different colors. This makes it possible to determine the end point of the titration without using a colored indicator.