Ion pair extraction titration
ON WHICH PRINCIPLE?
The more voluminous and hydrophobic the ions, the easier their extraction in an organic phase, since they will be solvated to a lesser extent by water molecules and will tend to solvate more with the organic solvent.
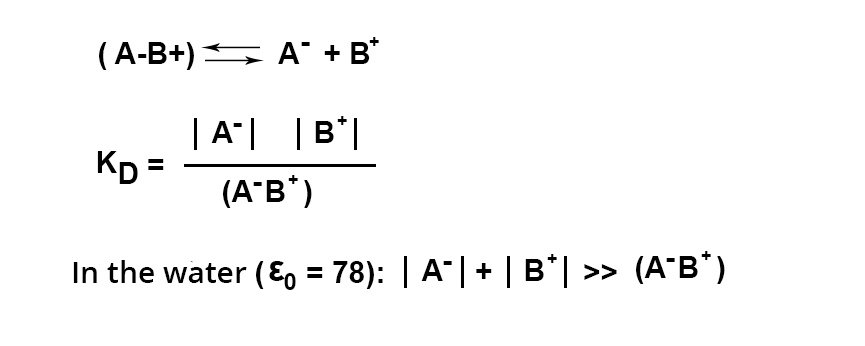
application to analysis
The extraction of a cation or anion in the form of an ion pair with a colored counterion is used for analytical purposes, provided that the extraction of the ion pair is quantitative.
This is only possible with an excess of dye. Excess that has not reacted during the formation of the ion pair must be insoluble in the organic phase at the working pH.
In pharmaceutics, it is usually amines and quaternary ammoniums (alkaloids, halogenated amine salts) that are extracted using anions from acid dyes.
Also, it is important to know the pKc of the acid giving the colored anion (A-), as well as the pKb of the base giving the cation (B+).




