Determination of fluoride ions using a selective electrode (potentiometry)
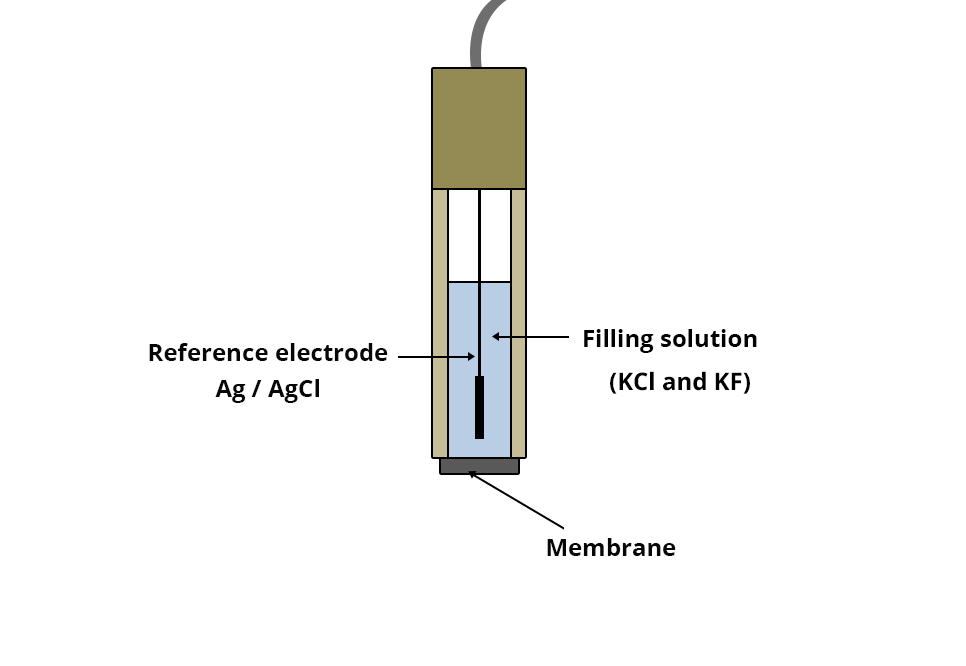
Constitution of the electrode
The crystalline electrodes selective to fluoride ions F- are made up of:
- A sealed membrane at the bottom of a plastic tube. This membrane is made of a thin layer of lanthanum fluoride monocrystal doped with europium fluoride (II) to enhance its conductivity.
- An internal reference electrode consisting of a silver wire coated with silver chloride (AgCl).
- An internal solution of KCl and KF.