How to perform a titration
Vitamin C titration
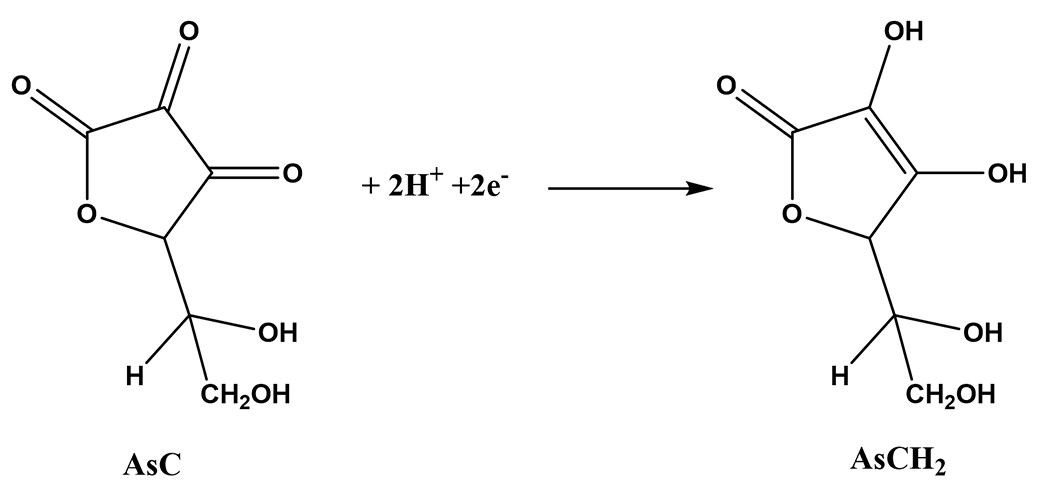
There are several methods of titration for the determination of vitamin C (ascorbic acid AsCH2). Three are presented here as examples.
Direct iodometric titration
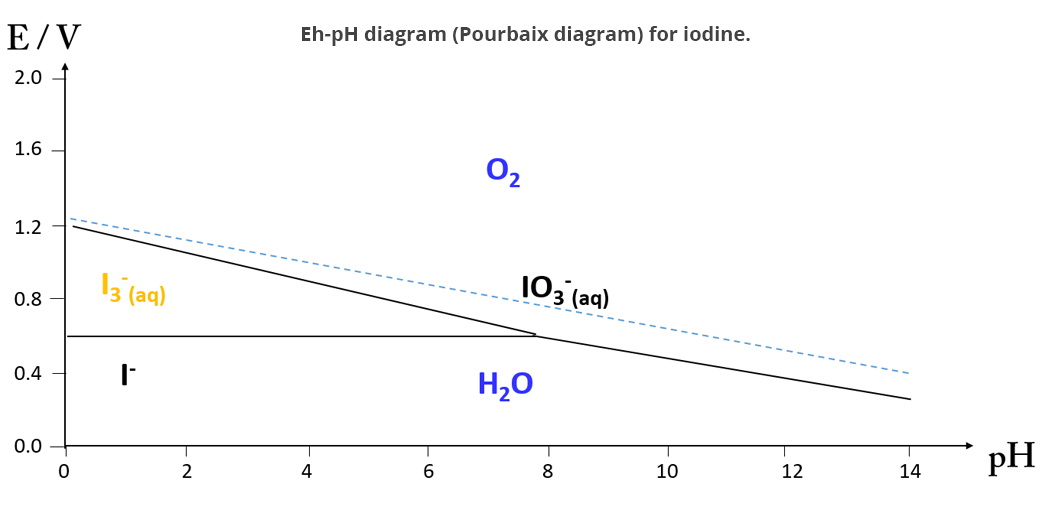
IO3- and I- are added to an acidic medium to form I3- (EH-pH diagram of the iode) which then reacts with vitamin C (ascorbic acid):
IO3-(aq) + I-(aq) = I3-(aq)
AsCH2(aq) + I3-(aq) = AsC(aq) + 3I-(aq)
As long as there is vitamin C, the solution is colorless.
When there is no more vitamin C, the solution turns orange, the color of I3-.
Indirect iodometric titration
An excess of I3- is added to the solution of ascorbic acid. The following reaction between I3- and ascorbic acid results in the formation of iodide ions I-:
AsCH2(aq) + I3-(aq) = AsC(aq) + 3I-(aq)
2S2O32-(aq) + I3-(aq) = S4O62-(aq) + 3I-(aq)
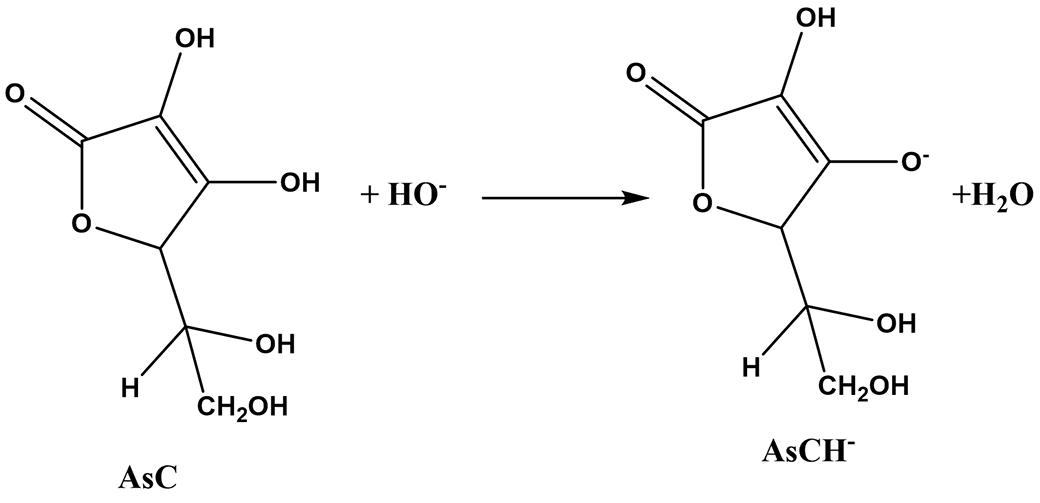
Acid-base direct colorimetry
Ascorbic acid can be dosed by pH-metric monitoring or by using cresol red as the colored indicator and lye as the titrant:
AsCH2(aq) + HO-(aq) = AsCH-(aq) + H2O(l)