THE CASE OF SULFAMETHOXAZOLE
ODtheo
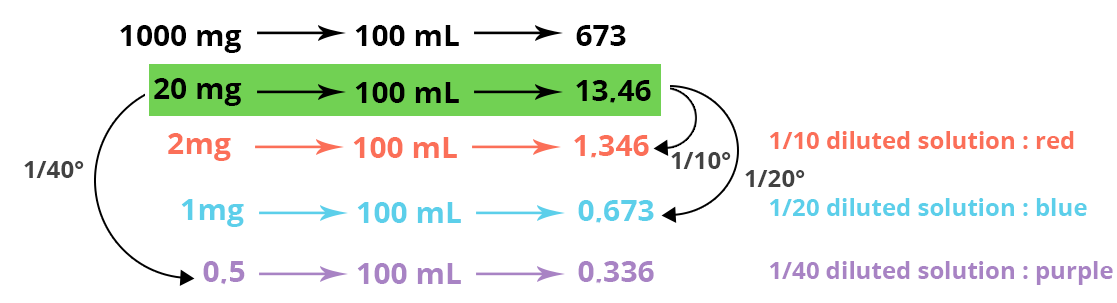
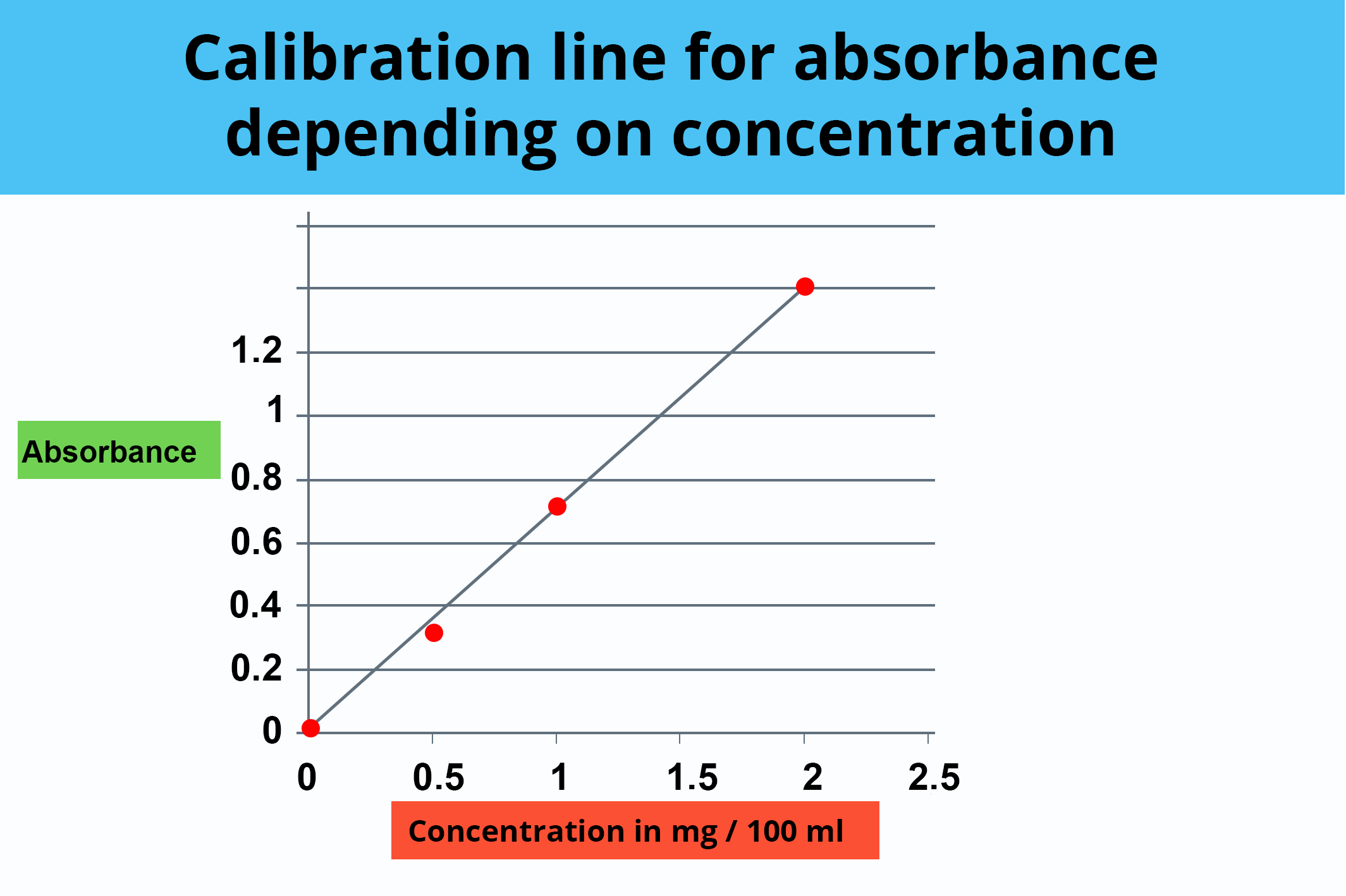
For example, for the preparation of the 1/10° solution, 10 mL of stock solution is taken and placed in a 100 mL volumetric flask and then filled to the top mark with basic water. When the diluted solutions have been prepared, the ODs are read on the spectrophotometer and the calibration curve can be constructed.
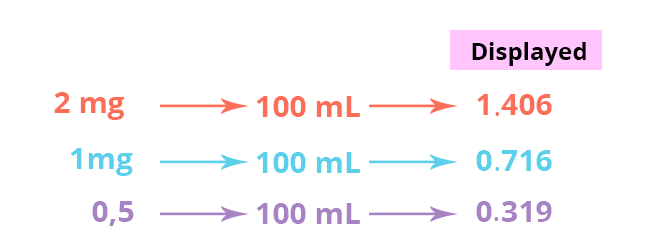
Here are the results: