Determination of dissolved oxygen with a Clark cell
How it works
Applying a potential difference pd (between 700 and 1200 mV), leads to electrolysis: the platinum (or gold) electrode acts as the cathode (reduction) and the silver electrode as an anode (oxidation). The reactions are:
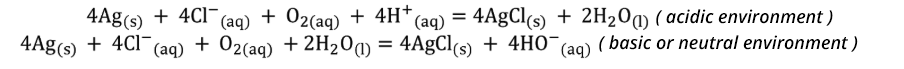
With a microampere meter, the cathodic current IC is measured. It gives the diffusion limit for the reduction reaction of O2 to HO- or H2O Iclim. The intensity of this current is directly proportional to the diffusion rate of O2 towards the cathode and the dissolved oxygen concentration [O2]:
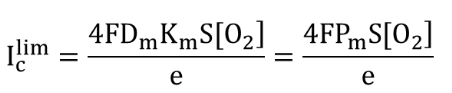
where F is the Faraday constant, Dm is the diffusion coefficient of O2 through the membrane, Km is the solubility product constant for O2 in the membrane, Pm is the membrane permeability which is DmKm, S is surface area of the electrode and e is the thickness of the membrane.
Dioxygen concentrations in the electrolyte solution and outside the cell are in equilibrium: if the dioxygen concentration in the solution increases, then that of the electrolyte solution increases as well. This makes it possible to determine O2 concentration by measuring the diffusion-limited current of the reduction reaction.