Determination of dissolved oxygen by colorimetric titration: the Winkler method
Dosing principle
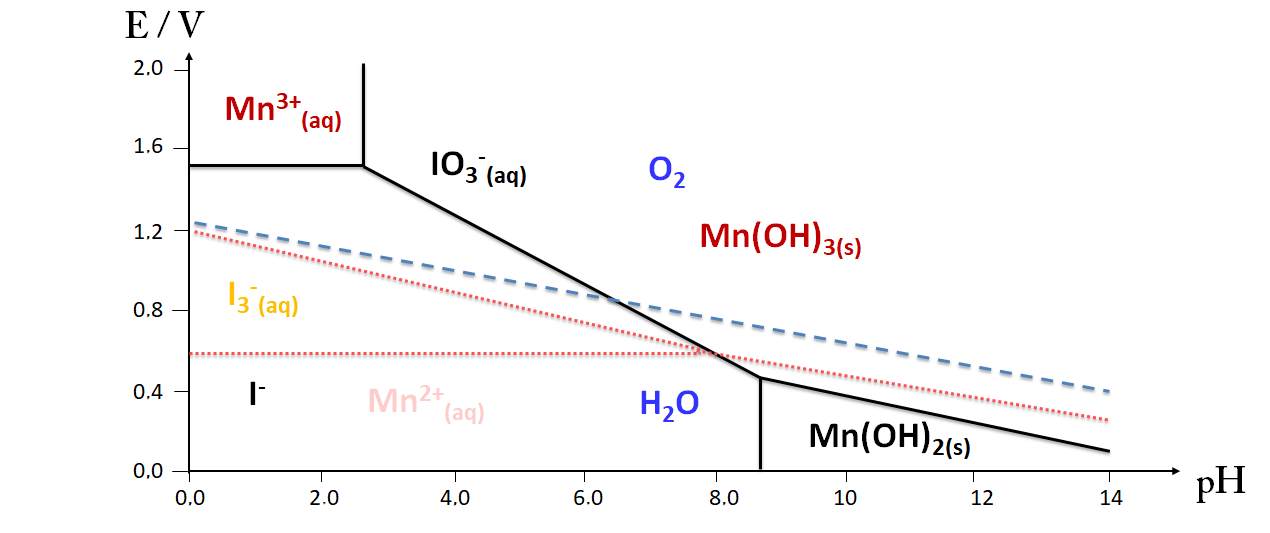
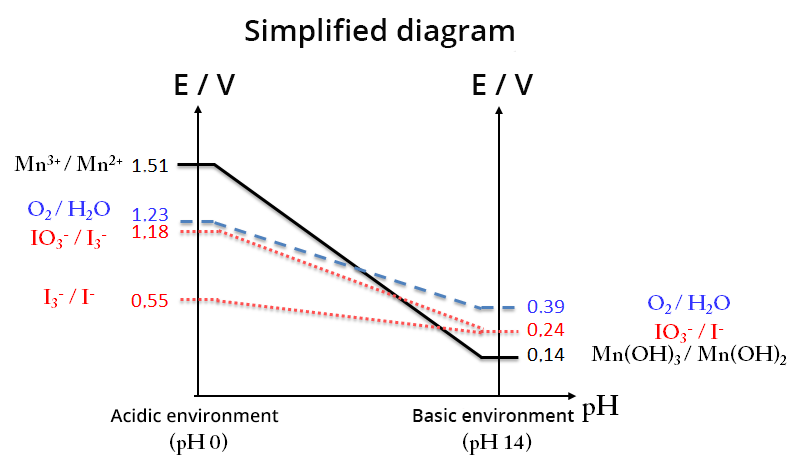
The determination of O2 concentration is based on the addition of MnCl2 to the water to be analyzed after it has been brought to a basic pH by the addition of sodium hydroxide pellets. Mn2+ ions at this pH precipitate as Mn(OH)2 hydroxide and react with the dissolved oxygen (1). A brown solid characteristic of Mn(OH)3 is formed. After a given time, we add sulfuric acid (2), then potassium iodide (3). Finally, the diiode is titrated with a solution of sodium thiosulfate (4).
(1) Mn2+(aq) + 2HO-(aq) = Mn(OH)2(s) and O2(aq) + 4Mn(OH)2 (s) + 2H2O(l) = 4Mn(OH)3(s)
(2) Mn(OH)3(s) + 3H+(aq) = Mn3+(aq) + 3H2O(l)
(3) 2Mn3+(aq) + 2I-(aq) = I2(aq) + 2Mn2+(aq)
(4) I2(aq) + 2 S2O32- (aq) = 2 I-(aq) + S4O62-(aq)
(2) Mn(OH)3(s) + 3H+(aq) = Mn3+(aq) + 3H2O(l)
(3) 2Mn3+(aq) + 2I-(aq) = I2(aq) + 2Mn2+(aq)
(4) I2(aq) + 2 S2O32- (aq) = 2 I-(aq) + S4O62-(aq)
Data:
pKS (Mn(OH)2) = 12.7; pKS (Mn(OH)3) = 36; E° (Mn3+/Mn2+) = 1.51 V;
E° (Mn(OH)3/Mn2+) = 2.01 V; E° (Mn(OH)3/Mn(OH)2) = 0.98 V; E° (I2 /I-) = 0.55 V;
E° (S4O62-/S2O32- ) = 0.08 V.
EH-pH diagram (Mn, I2 and H2O)