Determination of Cl- ions with a selective electrode (potentiometry)
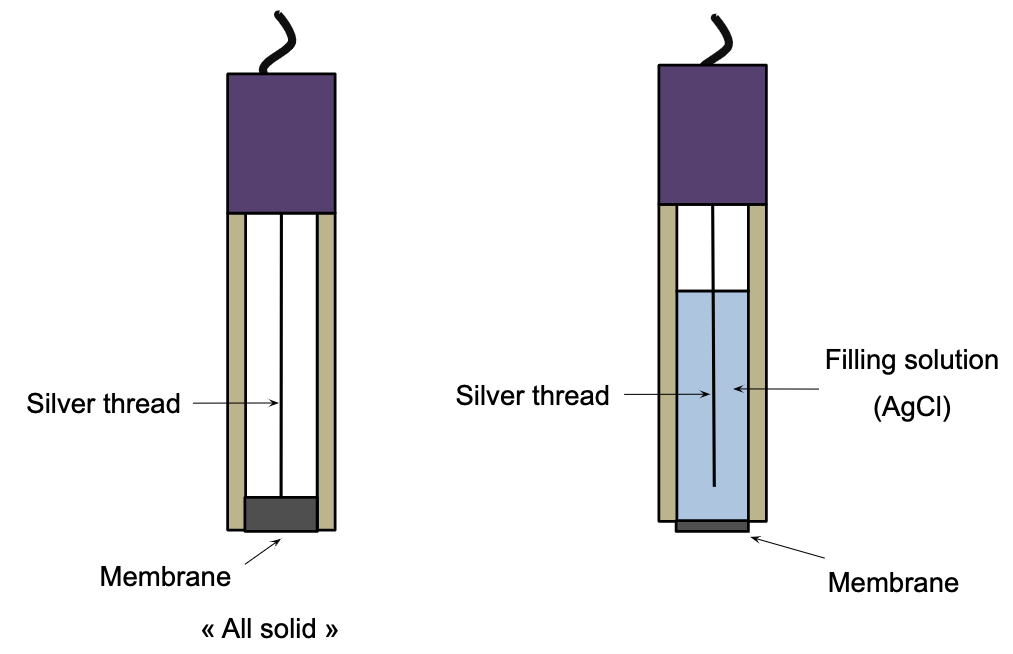
Electrode constitution
The selective electrode for chloride ions Cl– consists of:
- A pellet-shaped silver sulphide (Ag2S) and silver chloride (AgCl) poly-crystalline membrane situated at the end of a plastic cylinder.
- An internal reference electrode made of a silver wire which is inserted into the pellet.
Ag+ ions are able to migrate in the pellet (particularly in the external part). The potential of the electrode depends on the concentration of the Ag+ ions (and Cl- via the Ks of AgCl). This type of electrode is said to be “all solid”. Some selective electrodes for chloride ions containing a silver chloride solution are also available on the market.