How can the protein content of a bioproduct be determined?
The total protein content of a bioproduct can be determined by the Kjeldahl method. This method is applicable to any type of medium:
- food (solids, liquids)
- ingredients (liquid extracts, pastes, powders)
- vegetable and animal raw materials (cereals, insects)
On what principle is the Kjeldahl method based?
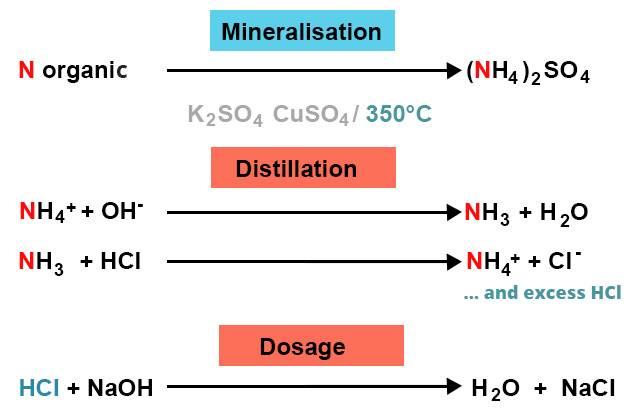
Nitrogen-containing organic compounds (proteins and nucleic acids in certain matrices) are heated and decomposed using sulfuric acid and a catalyst. This catalyst contains potassium sulfate (K2SO4), which increases the boiling temperature of sulfuric acid, and copper sulfate (CuSO4), which acts as a catalyst for the reaction. Nitrogen will quantitatively give ammonium sulfate: this is the mineralization stage.
The ammonia is subsequently displaced from its salt by sodium hydroxide, distilled by steam distillation and collected in a known quantity of excess hydrochloric acid. This step is called distillation.
The hydrochloric acid that has not reacted is dosed in return by sodium hydroxide. This is the dosing step.
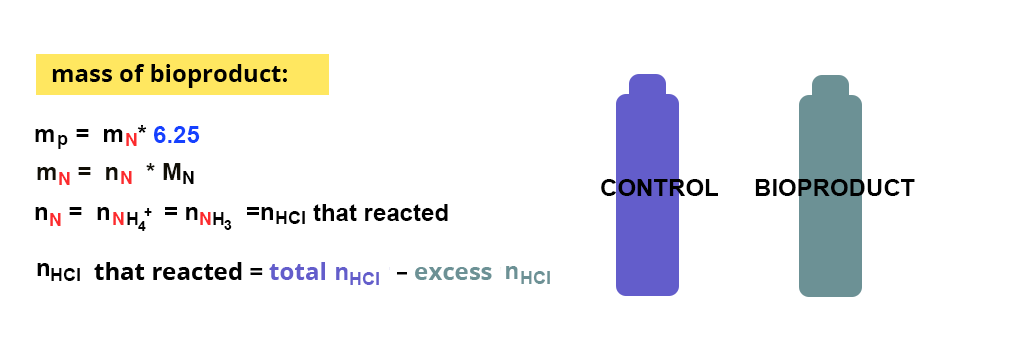
How can the protein content of a bioproduct be calculated?
Two samples will be prepared :
- a sample containing the bioproduct, liquid or previously ground
- a sample that does not contain the bioproduct, which will be the control
The following calculation will then be applied, considering a conversion factor of 6.25 (16% nitrogen on average in proteins):
 This experiment requires:
This experiment requires:
- suitable equipment (mineralizer, distiller, specific to the Kjeldahl method).
- suitable individual and collective protection (laboratory coat, gloves, glasses, fume hood).
- time: 2 half-days, part-time (not necessarily successive).




