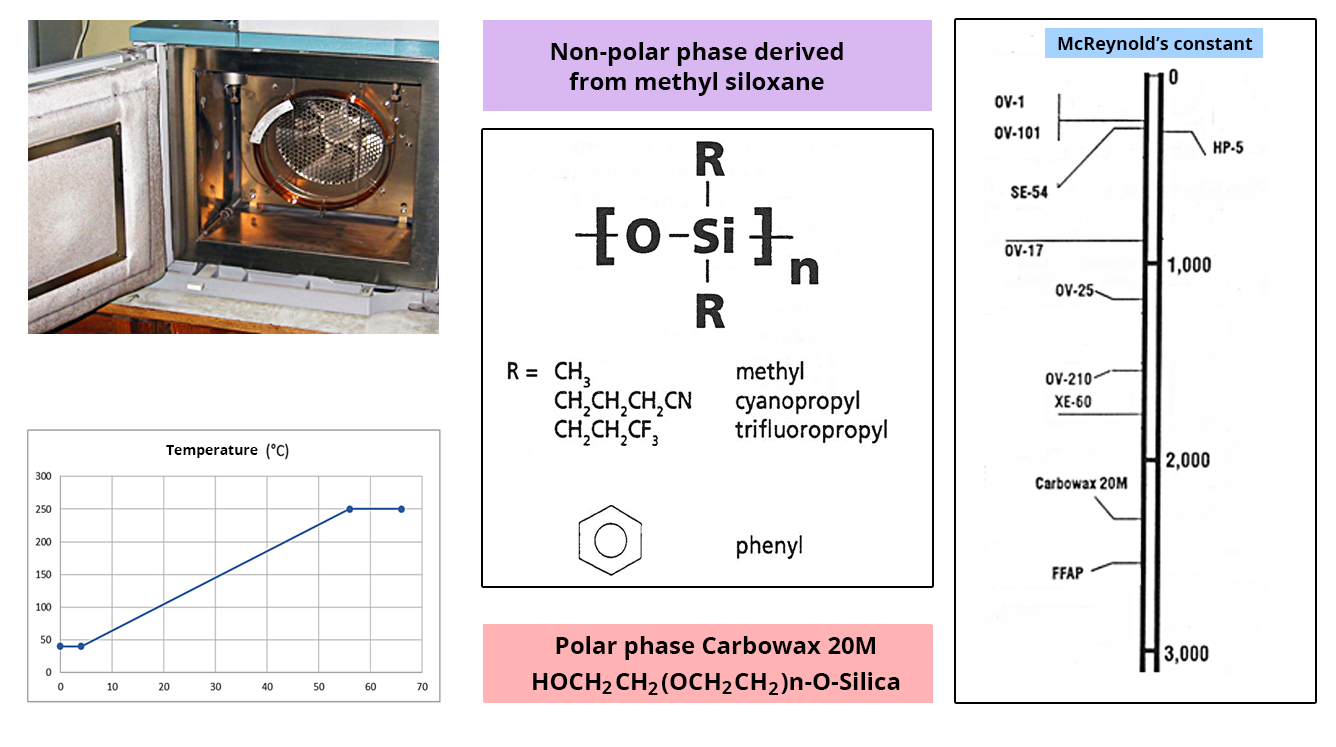
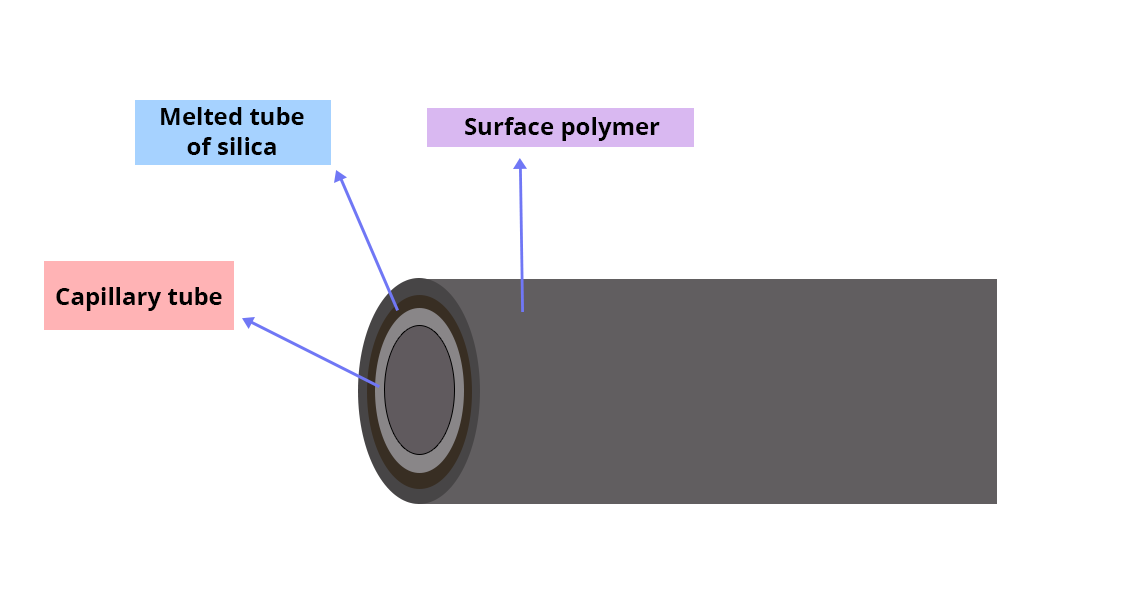
The separation column
This is a capillary tube with a very small diameter; its inner wall is coated with a certain layer of stationary phase.
Choosing the column:
- Small-diameter columns have a higher resolution but limit the thickness of the stationary phase.
- The thickness of the stationary phase is chosen in proportion to the volatility of the analytes that are to be separated.
The separation of the molecules takes place according to two phenomena:
- The volatility of molecules is defined by their boiling point. The temperature of the oven containing the column makes it possible to play with this parameter.
- Affinity for the stationary phase. The choice of the polarity of this phase is decisive for the separation.