Electronic transitions
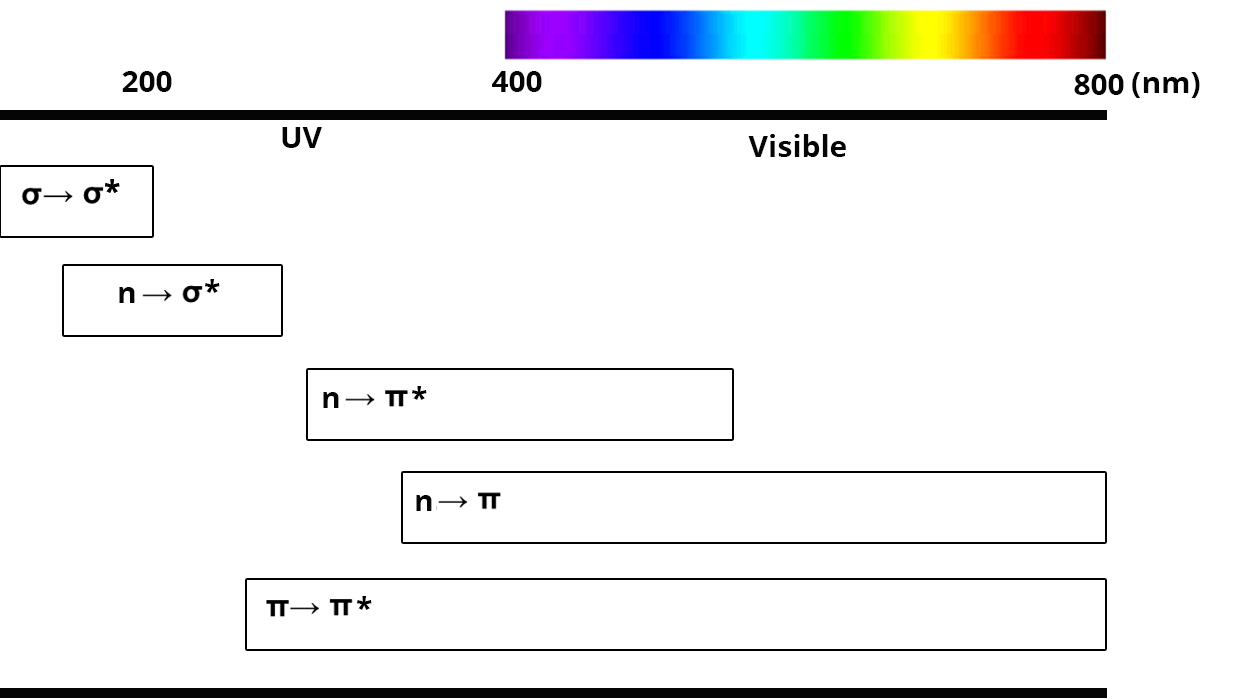
After excitation by electromagnetic radiation, valence electrons take part in electronic transitions. Only four different transitions are allowed between σ, π and lone pair electrons.
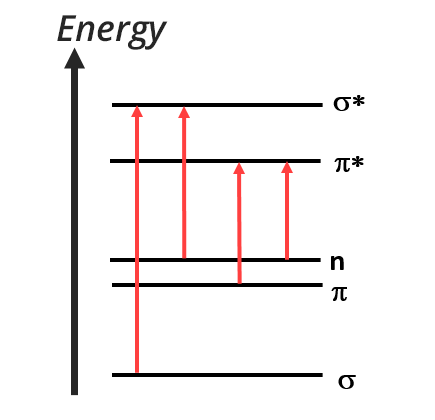
Transition σ → σ*
- occurs only for simple bonds (C-H or C-C for example)
- requires a high quantity of energy, and is thus found in the UV region (
Transition n → σ*
- occurs for atoms with lone pair electrons (N, O, S….)
- requires a high quantity of energy, and is thus found between 150 and 250 nm
Transition n → π* and n → π
- occurs only for atoms with lone pair electrons and for unsaturated functions
- there is sometimes a transition n → π when the level of the doublet is below the level of the π orbital
- these transitions usually take place in the UV domain
Transition π → π* (aromatics)
- occurs only for atoms with unsaturated functions
- are located in the UV and visible domain (200 to 700 nm)