What is a pH meter?
In order to measure pH, a measuring instrument sensitive to the hydronium ions that determine the pH value is required. This is a voltmeter.
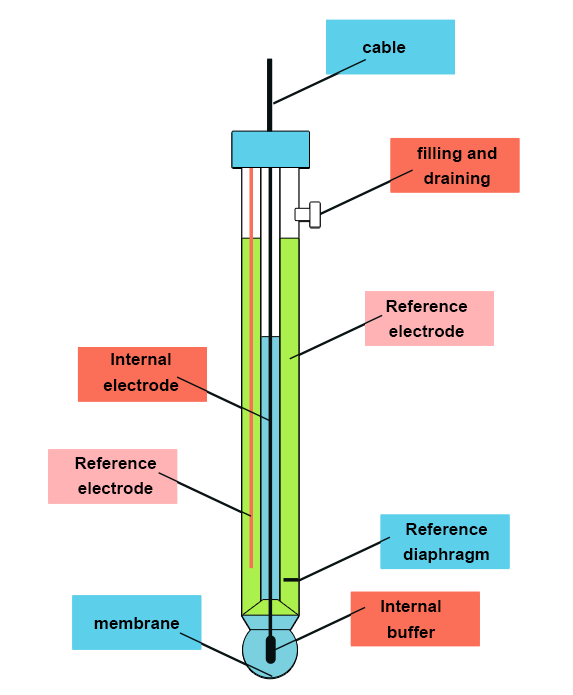
The measuring principle consists of taking a sensor with a glass membrane sensitive to hydronium ions (pH electrode) and observing the reaction between the membrane and the sample: measuring potential.
This potential is compared to a reference potential delivered by a non-pH-sensitive electrode, by diffusion of an electrolyte towards the sample through a diaphragm: reference electrode (Ag/AgCl).
The pH of a solution is therefore the potential difference between the two electrodes according to Nernst’s equation :
For simpler use, both electrodes are combined in one single electrode.
The measuring principle consists of taking a sensor with a glass membrane sensitive to hydronium ions (pH electrode) and observing the reaction between the membrane and the sample: measuring potential.
This potential is compared to a reference potential delivered by a non-pH-sensitive electrode, by diffusion of an electrolyte towards the sample through a diaphragm: reference electrode (Ag/AgCl).
The pH of a solution is therefore the potential difference between the two electrodes according to Nernst’s equation :
E = E0 + 2.3RT / nF * log [H3O+]
E = measured potential
E0 = constant
R = gas constant
T = temperature in degrees Kelvin
n = ionic charge
F = Faraday’s constant
E0 = constant
R = gas constant
T = temperature in degrees Kelvin
n = ionic charge
F = Faraday’s constant
For simpler use, both electrodes are combined in one single electrode.