THE CASE OF SULFAMETHOXAZOLE
Let’s consider the theory again to establish the calibration range:
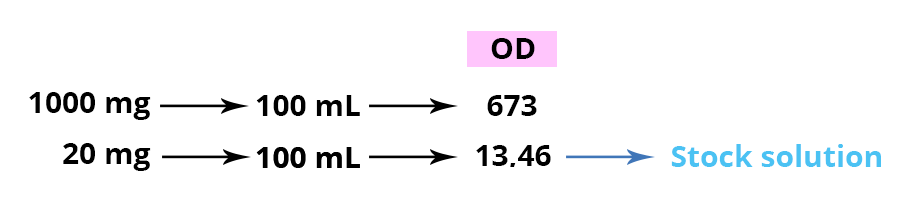
Specific absorbance of sulfamethoxazole is 673 in an alkaline solution at a λmax of 256 nM.
This implies that a solution of 1 g sulfamethoxazole in 100 mL (1%) of an alkaline solution should have an absorbance (or OD, Optical Density) of 673. This value cannot be measured with the usual equipment. It is therefore necessary to consider dilutions in order to fit within the analysis range of spectrophotometers which is around an OD of 1. Starting first with a 1/50 dilution:
Working solutions 1, 2, 3; corresponding to 1/10, 1/20 and 1/40 diluted solutions are prepared.




