UV spectrophotometry analysis
THE CASE OF SULFAMETHOXAZOLE
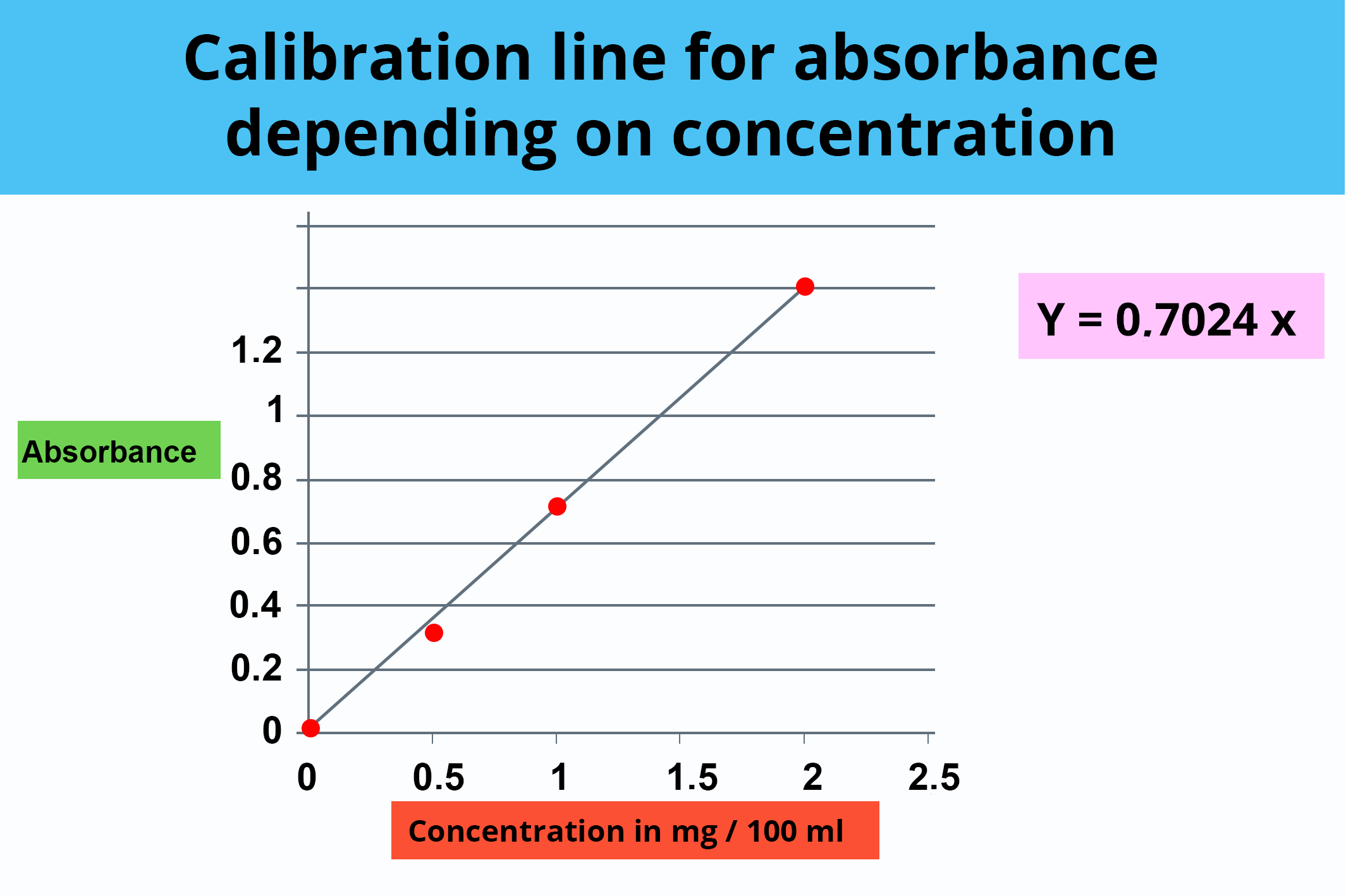
OD RM working solution = 0.712
OD Bactrim® powder working solution = 0.718
By plotting these values in the calibration range, the corresponding concentrations are obtained:
[RM working solution]: OD = 0.734 corresponding to a concentration of 1.04 mg/100 mL
[Bactrim® powder working solution]: OD = 0.718 corresponding to a concentration of 1.02 mg/100 mL
To restore the concentration of the respective stock solutions, the dilutions have to be “taken back up”. The working solution being 20 times less concentrated than the stock solution (1/20 dilution), multiplying by 20 gives:
[RM stock solution: 1.04 mg/100 mL x 20 = 20.9 mg in the initial 100 mL of stock solution.
By comparison with the 21 mg test sample, the purity of the raw material can be deduced: RM purity = 20.9/21 x 100 = 99.52% which complies with the pharmacopoeia.
[Bactrim® powder stock solution]: 1.02 mg/100 mL x 20 = 20.4 mg in the initial 100 mL of stock solution.
By comparison with the 27.4 mg test sample including 21.5 mg of sulfamethoxazole, the content of sulfamethoxazole in the tablet can be deduced:
Sulfamethoxazole content of Bactrim® = 20.4 mg in a 27.4 mg test sample corresponding to 379 mg in a tablet (509.4 mg) allegedly containing 400 mg sulfamethoxazole. If the approximate 5% error declared by the manufacturer is accounted for (380-420 mg) the tablet can be considered as compliant.




