Confirmation, quantification
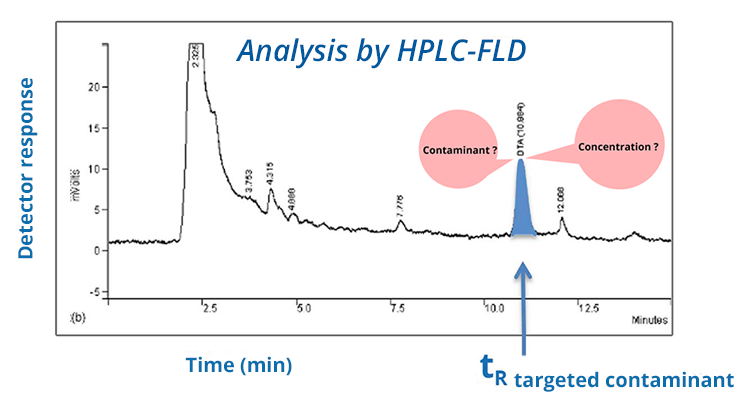
During the a priori investigation of a targeted contaminant, a chromatographic peak at the expected retention time of the contaminant may give you reason to suspect its presence. Nevertheless, this is not enough to establish its presence! The presence of the contaminant must be confirmed (because the peak can also be due to an interfering compound that co-elutes with the contaminant). To do so, two approaches may be considered, as indicated below. Once the peak attribution has been confirmed for the contaminant, quantification can be performed.
CONTAMINANT?
An analysis with a mass spectrometer as detector is the method of choice for confirming the presence of the target contaminant. In this case, one can:
- Either obtain the average mass spectrum associated with the chromatographic peak (or the mass spectrum associated with each retention time) if the detection is carried out in full scan mode (i.e. entire spectrum) – the comparison of this mass spectrum with the one expected for the contaminant confirms its presence (or not);
- Or follow certain ions characteristics of the contaminant: at least 2 are necessary, because the ratio of their relative abundances is a criterion for confirming the molecule (ideally 3 ions would be even better, if possible).
CONCENTRATION?
In chromatography, quantitative information is the area or the height of the chromatographic peak at the expected retention time for the contaminant of interest. In order to relate it to the contaminant concentration in the analyzed extract, a calibration is necessary (external, internal, or perhaps by standard additions if there is a matrix effect). The validity of the calibration should be checked periodically to ensure the reliability of the estimated concentrations.
Refer to the calibration sheet.